Many decapod crustaceans with sexually dimorphic body size are economically important species for aquaculture, making monosex culture a desirable practice to increase yields and profits.
Establishing monosexual populations in the giant freshwater prawn Macrobrachium rosenbergii, the same management approach has been attempted in commercially important penaeid shrimp, crabs and lobsters, without success.
There are many factors at play with species-specific complexities that require close examination when addressing monosexual production.
A team of researchers from the University of the Sunshine Coast has published a scientific review that provides a roadmap for successful sexual manipulation in decapod crustaceans, highlighting key issues and critical gaps in knowledge.
Meaning of sex manipulation in decapods
Sexual manipulation can be used for a variety of reasons depending on the species and the application. For aquaculture, the genus that provides the largest animals tends to be favored.
For example, female penaeid species grow faster and larger; therefore, an approach is sought to develop exclusively female populations.
Various investigations project that the successful application of sex change leads to a significant increase in income for shrimp farmers.
In the case of giant freshwater prawn, research has reported that mixed and female-only populations grow 1.5 to 2 times more slowly than male-only populations achieved by hand sorting.
Currently, culture of monosex populations of M. ronsebergii has been developed using the proprietary iag10 gene silencing technique and essential genetic markers of sex to validate successful sexual manipulation.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
Sexual manipulation in decapods is necessary to achieve higher performance, higher profits, or higher aquaculture productivity.
In addition to applications in the aquaculture industry, monosexual populations of decapods can be used to control carriers of human pathogens; Because monosexual populations cannot reproduce, they can be used safely as a biocontrol agent in West African countries for schistosomiasis infestations and also to reduce the risk of invasion by invasive alien species.
Biotechnological approach to monosex in decapods
While the concept of monosexual culture of crustaceans was shown to be feasible by manual segregation, it was not until research identified genetic markers for sex and iag that this became a commercial reality, indicating that the development of a sustainable monosex farming requires the establishment of efficient biotechnology.
In M. rosenbergii, monosexual production is a common practice today, due to the establishment of sexual genetic markers and iag-directed gene silencing; this is done early in development, before the establishment of sexual characteristics.
Farmers using the proprietary technology report a significant increase in income because M. rosenbergii males grow larger and faster than females.
Key aspects to consider
Androgen gland
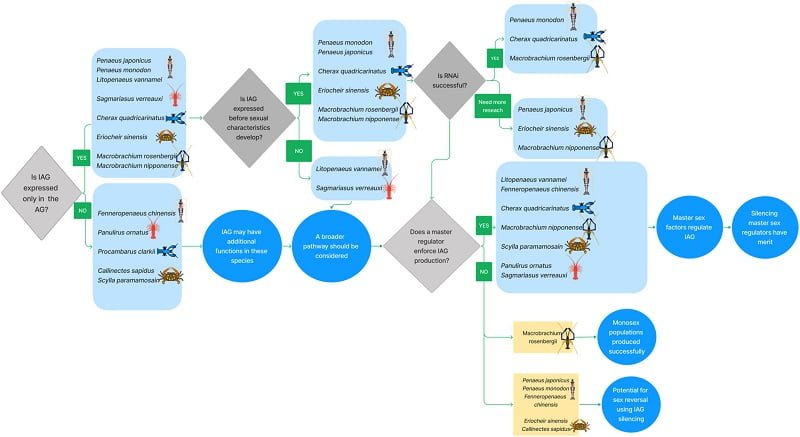
The specific androgen (AG) gland of male river shrimp is known to mediate masculinization by producing and secreting the insulin-like hormone AG (IAG).
The spatial pattern of iag expression has been reported to vary among decapod species. In some decapods, iag is expressed only in the AG, while in other species iag is expressed in multiple tissues.
However, in M. rosenbergii, where iag manipulation is successful, iag expression was detected exclusively in the AG. This observation could explain why AG manipulation led to complete sex change in M. rosenbergii and perhaps could explain why it failed in other species.
Since successful sex change has not been achieved to date in any decapod species other than M. rosenbergii, more aspects need to be considered, as detailed below.
When does the iag expression start?
It is well established that IAG regulates the development of male primary and secondary sexual characteristics. It is clear, however, that IAG is not a determinant of sex.
When iag is silenced in these intersex individuals, the arrested ovary matures and produces vitellogenin, while spermatogenesis regresses, shifting the balance toward a female phenotype.
Is RNA interference (RNAi) gene silencing effective?
The use of RNAi for sexual manipulation in decapod crustaceans is a very practical application in aquaculture. In vivo silencing by injection of freshwater prawn, M. rosenbergii, with Mr-iag-specific dsRNA, at an early stage of development in young males, induced a complete sex and functional switch from genetic males to neo-females. .
In addition, in the tiger shrimp, P. monodon, and the Australian crayfish, C. quadricarinatus, RNAi technology has been successfully applied for gene manipulation. However, in several other decapod species, RNAi technology did not work efficiently.
Other avenues of sexual manipulation
Other means of endocrine manipulation have attracted considerable attention and have had some success in skewing the sex ratio in crustaceans. The sex ratio in juvenile M. rosenbergii was significantly altered by feeding them lipid-enriched Artemia with 17a-methyltestosterone (MT).
In addition, temperature, salinity, light, and other factors have been shown to affect sex determination and differentiation in some crustaceans. Cold shock induces triploidy and changes the sex ratio in P. monodon, while daylength influences the sex ratio in Gammarus deubeni.
Another promising path to successful sexual manipulation involves genome editing in crustaceans. First decapod genome editing deleted a gene that regulates eye formation in the eastern shrimp Exopalaemon carinicauda using CRISPR/Cas9 microinjected into embryos
Conclusion
We provide here a detailed roadmap for successful sexual manipulation in decapod crustaceans, highlighting key caveats to consider and critical gaps in current knowledge.
They recommend taking into account the timing of iag expression in relation to the development of sexual characteristics, the relationship between IAG and a master sexual regulator, as well as gene silencing capacity.
“To date, a large number of genes regulating sexual development in non-decapods have been elucidated, greatly facilitated by cutting-edge DNA and RNA sequencing technologies and bioinformatics analysis,” they conclude.
The study was funded by the Australian Research Council and the University of the Sunshine Coast.
Contact
Tomer Ventura
Centre for Bioinnovation
University of the Sunshine Coast,
Sippy Downs, Queensland, 4556, Australia.
Email: tventura@usc.edu.au
Reference (open access)
Nguyen, AHT, Glendinning, S, Ventura, T. A refined roadmap to decapod sexual manipulation. Rev Aquac. 2023; 1- 10. doi:10.1111/raq.12808
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.







