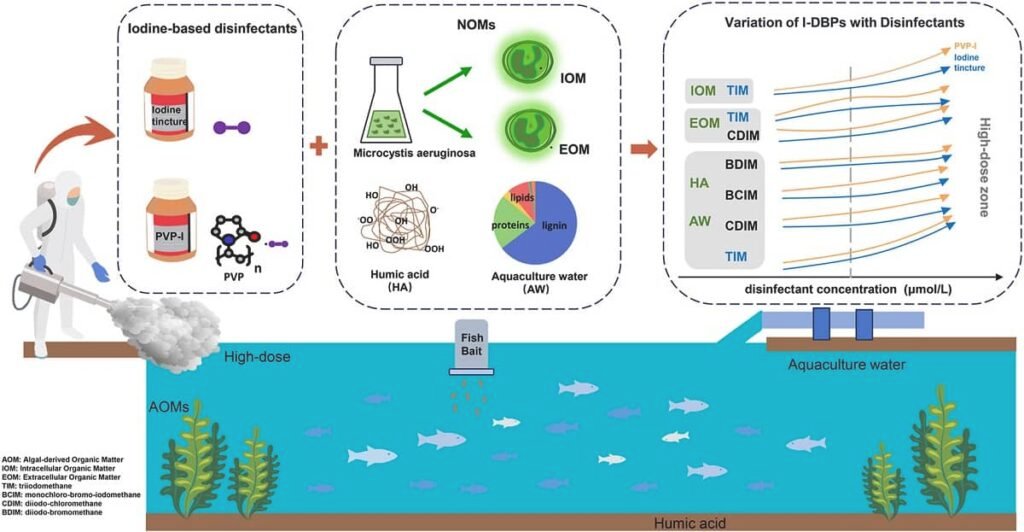
In modern aquaculture, particularly in intensive systems, the use of disinfectants is a fundamental tool for preventing and controlling diseases that threaten the health of fish and shrimp. Among the most common are iodine-based disinfectants, such as iodine tincture and povidone-iodine (PVP-I), which are valued for their efficacy and low cost.
However, their application is not without consequences. When these disinfectants come into contact with the natural organic matter in the water (e.g., leftover feed, excrement, algae), they can trigger chemical reactions that create undesirable compounds: iodinated disinfection byproducts (I-DBPs). These compounds have raised concern because studies indicate they possess higher cytotoxicity and genotoxicity compared to their chlorinated and brominated counterparts.
A recent study by scientists at Tongji University, published in the scientific journal Chemosphere, delves into this phenomenon by investigating how and under what conditions these I-DBPs form within the context of aquaculture. The findings offer valuable guidance for optimizing disinfection practices and minimizing the associated environmental risks.
Key conclusions
- Iodine-based disinfectants, such as iodine tincture and povidone-iodine (PVP-I), can react with organic matter in farm water to form potentially toxic iodinated disinfection byproducts (I-DBPs).
- Maintaining the water’s pH at a slightly alkaline level, around 8.0, is an effective strategy to significantly reduce the formation of these harmful byproducts.
- The type of organic matter present in the water is crucial. Humic acid (common in waters with waste and plant material) has a much higher potential to generate I-DBPs compared to organic matter derived from algae.
- Higher disinfectant doses lead to an exponential increase in I-DBP formation. Povidone-iodine (PVP-I) requires a higher concentration than iodine tincture to begin generating byproducts due to its slower release of iodine.
How was the study conducted?
To understand I-DBP formation, the researchers simulated aquaculture pond conditions in a laboratory setting. They used the two most common iodinated disinfectants: iodine tincture and povidone-iodine (PVP-I).
The experiment involved reacting these disinfectants with different organic “precursors” typically found on aquaculture farms:
- Humic Acid (HA): A model for organic matter derived from the decomposition of plants and other materials, which is very common in natural waters.
- Algal Organic Matter (AOM): Both extracellular organic matter (EOM), released by algae during growth, and intracellular organic matter (IOM), released when they die and decompose, were extracted.
- Aquaculture Pond Water: Samples were taken from a sea bass culture pond to observe how the disinfectants behaved in a complex and realistic environment.
The scientists evaluated key factors, such as disinfectant concentration and water pH, to determine their impact on the quantity and type of I-DBPs generated.
Factors determining I-DBP formation
Water pH: A crucial control actor
Water pH proved to be a determining factor. The study revealed that maintaining the pH at a slightly alkaline level, near 8.0, effectively reduces I-DBP formation. Under acidic conditions, the formation of iodoform (TIM), the predominant iodinated byproduct, was considerably higher, especially with iodine tincture. This suggests that a simple adjustment and monitoring of pH can be a first line of defense in mitigating the generation of these compounds.
Dose and type of disinfectant: More is not always better
The disinfectant concentration has a direct and exponential impact. At low doses, I-DBP formation was minimal or non-existent. However, as the concentration was increased to simulate a “high-dose” scenario, the quantity of byproducts grew multiplicatively.
A significant difference was observed between the two disinfectants:
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
- Iodine tincture: Began producing TIM at a relatively low concentration (2.5 µM).
- Povidone-iodine (PVP-I): Required a four times higher concentration (10 µM) to start generating TIM. This is because PVP-I is a complex that releases iodine more slowly and in a controlled manner.
At very high concentrations, not only did the amount of TIM increase, but other types of I-DBPs also appeared, such as BCIM (bromochloroiodomethane), CDIM (chlorodiiodomethane), and BDIM (bromodiiodomethane).
Organic matter: Not all precursors are equal
The type of dissolved organic matter in the water was, perhaps, the most revealing factor.
- Humic Acid (HA): Demonstrated the highest potential for forming I-DBPs. Its aromatic structure is highly reactive with iodine, facilitating the creation of a greater quantity and variety of byproducts.
- Algal Organic Matter (AOM): In comparison, organic matter derived from algae generated fewer I-DBPs. Nonetheless, differences were found among its components:
- Extracellular matter (EOM) tended to produce a greater variety of I-DBPs.
- Intracellular matter (IOM), especially at high disinfectant concentrations, contributed significantly to TIM formation. This is because IOM contains larger molecules with more reactive carbon available for iodine substitution.
The real-world scenario: Disinfection in fish farm water
When the researchers experimented with real water from an aquaculture pond, the results were very similar to those obtained with humic acid. An analysis of the pond water revealed that its organic matter contained humic-like components, such as lignin and proteins, likely originating from fish feed and excrement.
This confirms that in a real farming environment with a high organic load, the risk of I-DBP formation is significant, especially when high doses of disinfectants are used.
Conclusion: Towards safer and more efficient disinfection
This study underscores that while iodine disinfectants are effective, their use must be carefully managed to avoid water contamination with potentially toxic byproducts. The formation of I-DBPs is not inevitable and can be controlled.
For aquaculture producers, the main lesson is that optimizing disinfection requires a comprehensive approach that considers water chemistry. Controlling the pH to maintain it around 8.0, adjusting disinfectant doses to avoid overuse, and understanding the pond’s organic matter load are key strategies to minimize I-DBP formation and protect both the health of the cultured organisms and the environment.
Contact
Xialin Hu
Key Laboratory of Yangtze River Water Environment, Ministry of Education, College of Environmental Science and Engineering, Tongji University
1239 Siping Road, Shanghai, 200092, PR China.
Email: xlhu@tongji.edu.cn, xialin.hu@gmail.com
Reference
Wen, D., Wang, J., Ding, M., Zhamaerding, A., Hu, X., & Yin, D. (2025). Formation of iodinated disinfection by-products from high-dose disinfection with two types of iodine disinfectant in aquaculture. Chemosphere, 387, 144653. https://doi.org/10.1016/j.chemosphere.2025.144653
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.







