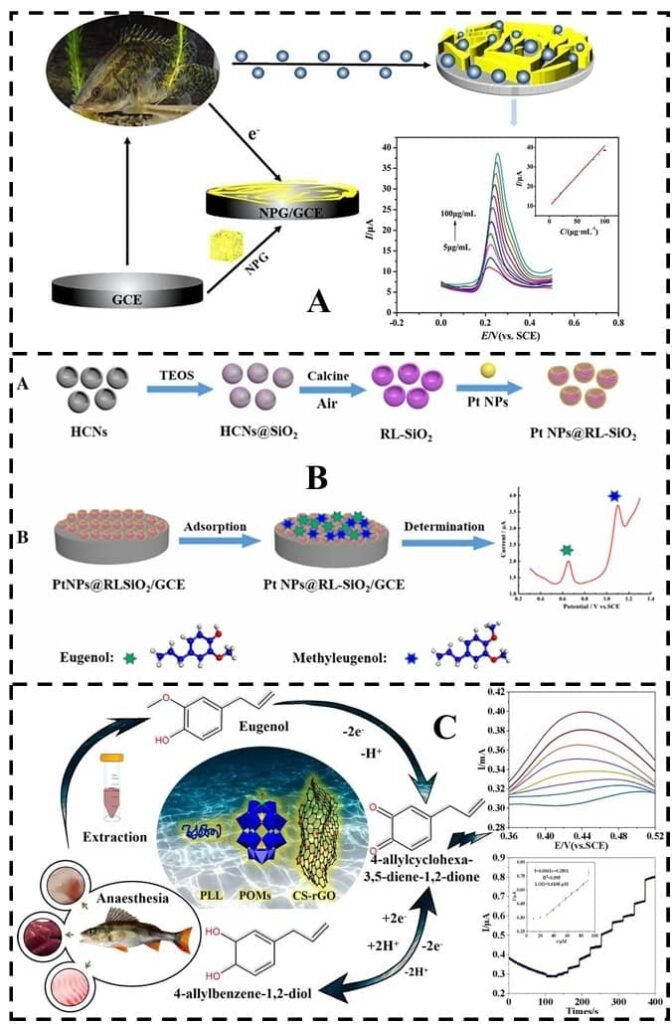
Fresh aquatic products are a vital source of protein and essential nutrients in the human diet, valued for their flavor and low cholesterol content. However, as the market expands, the safety of aquaculture products has become a primary concern, particularly regarding chemical residues.
In this context, anesthetics used in aquaculture play a fundamental role. Routine procedures such as handling, sampling, sorting, and transport can induce intense stress in fish, leading to injuries, high mortality, and a decrease in product quality. The judicious use of anesthetics mitigates these adverse effects, reduces economic losses, and supports animal welfare.
Nonetheless, their application presents a significant challenge: a lack of regulation. In many regions, dosages and withdrawal periods (the time required for the chemical to be eliminated from the organism before consumption) are determined empirically. This, combined with regulatory disparities between countries, increases the risk of anesthetic residues remaining in edible tissues, raising concerns about human health and environmental impact.
A study published by researchers from Guangdong University of Education, South China Agricultural University, and the Guangzhou Institute of Food Inspection analyzes six common anesthetics in aquaculture (eugenol, MS-222, benzocaine, 2-phenoxyethanol, diazepam, and quinaldine), their associated risks, and the analytical methods for their detection, based on a recent scientific review.
Key conclusions
- Anesthetics such as eugenol, MS-222, and benzocaine are crucial for reducing stress, injuries, and mortality during the handling and transport of fish.
- The lack of harmonized regulations, empirical use of dosages, and differing withdrawal periods increases the risk of hazardous residues in aquaculture products.
- The anesthetic MS-222 is the only one approved by the FDA in the U.S., but only for specific fish families (such as salmonids and ictalurids) and with a strict 21-day withdrawal period.
- The use of sedatives like diazepam is prohibited in food-producing animals in the EU and China, but its illegal use persists, presenting a serious risk due to its highly persistent residues in fish.
- Rapid detection methods (immunoassays and biosensors) and the development of new, eco-friendly anesthetics are needed to ensure consumer safety and industry sustainability.
Anesthetics under scrutiny: A detailed analysis
Anesthetics are chemical agents that induce sedation or temporary immobility. Their selection depends on the species, the operation, and safety considerations. The most commonly used anesthetics and their safety profiles are detailed below.
Eugenol (Clove Oil)
Eugenol is a natural phenolic compound, the main component of clove oil (85-95%).
- Action and benefits: It induces temporary immobility, reduces physical injuries (such as scale loss), and minimizes the risk of infections. It also decreases ammonia excretion, improving water quality during transport.
- Residues and safety: It is absorbed through the gills and rapidly eliminated. A study in white shrimp (Litopenaeus vannamei) showed that residues dropped to safe levels within 24.5 hours. Water temperature is key: a faster metabolism (and shorter elimination time) is observed at higher temperatures (e.g., 2 hours at 20°C vs. 4 hours at 13°C in sea bass).
- Risks: In high concentrations, it can be cytotoxic, causing abnormalities in zebrafish embryos.
- Regulation: Varies greatly. It is approved in New Zealand, Japan (MRL of 50 ng/mL and a 7-day withdrawal), Australia, and Chile. However, it is not approved as a fish anesthetic in the United States or China (though the US FDA permits it as a food additive).
MS-222 (Tricaine)
MS-222, or tricaine, is one of the most widely used anesthetics globally due to its rapid action and recovery.
- Action: It acts by blocking sodium ion channels in muscle and nerve cells.
- Residues and safety: It is absorbed through the gills and skin and is metabolized mainly in the liver and gills. It generally does not accumulate in tissues. Its efficacy is affected by water temperature, pH, and salinity. It is preferred for cold-water species, such as salmonids.
- Risks: Human consumption of fish with high residual concentrations can cause skin and respiratory irritation, and in rare cases, retinal damage.
- Regulation: It is the only anesthetic approved by the US FDA for aquaculture. However, its use is restricted to specific families (Ictaluridae, Salmonidae, Esocidae, and Percidae) and requires a 21-day withdrawal period. It is also approved in Norway (21-day withdrawal) and New Zealand (10 days). It is not approved in China or the European Union.
Benzocaine
Benzocaine is a lipophilic compound with rapid action and low toxicity, making it a cost-effective alternative.
- Action: Blocks sodium channels, preventing the transmission of nerve impulses.
- Residues and safety: It is metabolized and excreted mainly through the gills (59.2% in 3 hours in rainbow trout), and to a lesser extent via the kidneys and bile.
- Risks: Although generally safe for fish, excessive human exposure through residues can cause arrhythmias, coma, and allergic reactions.
- Regulation: The US FDA has an import tolerance of 50 µg/kg. Australia and New Zealand have established an MRL (Maximum Residue Limit) of 0.05 mg/kg. China has not yet established specific regulations for its use.
Prohibited and high-risk anesthetics
Not all sedatives are safe or legal for use in fish intended for human consumption.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
2-Phenoxyethanol
Although low-cost and possessing bactericidal properties, 2-phenoxyethanol presents serious concerns.
- Risks: It poses residual problems, its effect is prolonged, and it can be harmful to fish. Experts recommend avoiding its application in aquaculture species intended for human consumption.
- Regulation: Its use in fish for human consumption is prohibited in both the United States and the European Union. Its application is restricted to ornamental or research fish.
Diazepam
Diazepam is a long-acting benzodiazepine sedative, not a traditional anesthetic. It has been used illegally to reduce the metabolic rate of fish, alleviate stress, and improve survival rates.
- Risks: The main danger is that its residues are persistent and can be transferred to humans through the food chain. Concentrations up to 118.6 µg/kg have been detected in freshwater fish. A study in Carassius auratus (goldfish) showed a half-life of 619 hours in muscle and skin, requiring at least 70 days for the concentration to fall below the quantifiable limit. Human consumption can cause fatigue, drowsiness, mental confusion, and, in severe cases, coma or carcinogenic effects.
- Regulation: Its use in food-producing animals is prohibited in many countries, including China (Announcement No. 193) and the European Union (Regulation (EC) No 470/2009).
Quinaldine
This is an alkaloid anesthetic used since the 1950s.
- Risks: Research on its pharmacokinetics (how it moves through the body) and toxicology is very limited. Furthermore, wastewater containing quinaldine is resistant to degradation, posing ecological risks.
- Regulation: Currently, no national or international authority explicitly authorizes the use of quinaldine in fish for human consumption.
How are anesthetic residues detected?
Monitoring residues is essential but complex. Fish components (proteins, fats) can interfere, and residues are often present in very low concentrations.
Instrumental methods (Laboratory)
These methods are highly accurate and sensitive, although they require time and specialized personnel.
- Gas Chromatography (GC and GC-MS): These are the preferred methods due to their high sensitivity. They are especially effective for volatile or semi-volatile anesthetics, such as eugenol and MS-222.
- Liquid Chromatography (LC and HPLC): Unlike GC, these do not require the compound to be volatilized. They are better suited for non-volatile or thermally unstable anesthetics, such as 2-phenoxyethanol and benzocaine.
Rapid detection methods (Field)
For the rapid analysis of large quantities of samples in situ, alternatives to laboratory instruments are being developed.
- Immunoassays (ELISA, GICA): These are based on the specific antigen-antibody reaction. Methods like ELISA or colloidal gold immunochromatography strips (GICA) allow for rapid and sensitive detection, sometimes visible to the naked eye.
- Electrochemical sensors: These convert the chemical properties of the anesthetic into an electrical signal. They offer high sensitivity, ease of operation, and potential for miniaturization for field use.
Conclusions and the future of anesthesia in aquaculture
Anesthetics like eugenol, MS-222, and benzocaine are essential tools for improving animal welfare and reducing losses in aquaculture. However, the lack of harmonized global regulations and concerns about residue accumulation in edible tissues remain critical challenges.
The situation is complicated by the illegal use of prohibited sedatives like diazepam, which, due to its high persistence, represents a direct risk to food safety.
To move forward, the sector needs to prioritize research in several key areas:
- The development of new, eco-friendly anesthetics with low toxicity and rapid biodegradation to reduce residue accumulation.
- Advancements in multi-residue detection technologies, such as more sensitive biosensors and point-of-care testing, to enable faster and more efficient monitoring.
- The adoption of a “One Health” approach, integrating human, animal, and environmental health, to create globally harmonized regulatory frameworks.
Reference (open access)
Jia, B.-Z., Rui, X.-Y., Wang, Y., Zeng, X., Sheng, S.-J., Zeng, B.-J., Xu, Z.-L., & Luo, L. (2025). Fishery Anesthetics in Aquaculture Products: Safety Concerns and Analytical Methods. Foods, 14(22), 3928. https://doi.org/10.3390/foods14223928
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.




