Tilapia, a popular fish in commercial production, is particularly vulnerable to infections caused by species of Aeromonas and Streptococcus. These pathogens can have devastating effects on fish populations and profitability.
A team of researchers from the Federal University of Latin American Integration, Alquimia Pescados, and the Federal University of Espírito Santo have developed and analyzed a bivalent vaccine against Streptococcus agalactiae and Aeromonas hydrophila, two of the main pathogens affecting tilapia.
Fish farmers now have a new tool in the form of an innovative bivalent vaccine designed to protect Nile tilapia (Oreochromis niloticus) from these pathogens.
The Vaccine Breakthrough
Researchers have developed a bivalent vaccine designed to safeguard Nile tilapia against two of the primary culprits of diseases: A. hydrophila and S. agalactiae.
To prepare the vaccine, researchers inactivated the cultures by adding 10% buffered formalin to a final concentration of 3% in an incubator with agitation at 100 RPM (rotations per minute) for 24 hours at 25°C.
The vaccine was administered through intraperitoneal (ip) injection, and its effectiveness was rigorously tested in two phases.
Laboratory Success
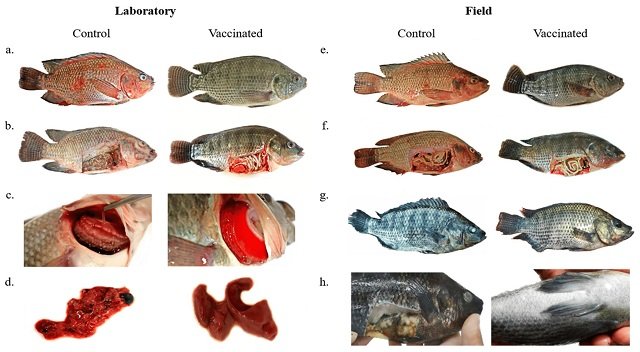
In the initial laboratory tests, the bivalent vaccine demonstrated remarkable safety and efficacy. The vaccine achieved an impressive vaccine efficacy (VE) of 93.66%, showcasing its potential to protect fish from these harmful pathogens.
Encouraged by the laboratory findings, the study expanded to large-scale tests involving 12,000 tilapias. In this real-world setting, the vaccine’s VE remained commendable at 59.14%.
While survival may appear lower than the laboratory result, it is important to note that the vaccinated group outperformed the control group in feed conversion. Vaccinated tilapias had a feed conversion of 1.27 kg, whereas the control group had a feed conversion of 1.54 kg.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
“In field experiments, vaccinated animals showed greater growth than the control animals. Both groups exhibited a similar feeding pattern for the first three months; however, in the end, the vaccinated group had approximately 22% more body mass per animal compared to the control group,” the researchers reported.
These results not only affirm the vaccine’s effectiveness but also highlight its potential economic benefits for fish farmers.
The Road Ahead
The successful development and evaluation of this bivalent vaccine against Aeromonas and Streptococcus species represent a significant step forward in the field of aquaculture. While the results obtained so far are promising, there is still work to be done.
Field tests in real production settings are the next logical step to confirm the vaccine’s real-world protective capabilities.
Conclusion
The potential of this bivalent vaccine to protect Nile tilapia from pathogens is a ray of hope for tilapia fish farmers. As global demand for aquaculture products continues to rise, innovations like this vaccine are essential for ensuring sustainable and profitable fish farming.
“Overall, immunization with a bivalent vaccine against A. hydrophila and S. agalactiae administered via i.p. injection proved to be safe and effective in reducing mortality in fish raised in high-density production tanks,” the researchers conclude.
With further field tests and refinement, this vaccine could become a cornerstone in the arsenal of tools available to fish farmers, offering greater protection and prosperity in commercial aquaculture production.
However, researchers recommend conducting field tests to confirm the vaccine’s real-world protection.
Currently, there is a patent for this invention titled “Bivalent Vaccine for Tilapia” (BR1020190263644), filed with the National Institute of Intellectual Property (INPI). Additionally, the study has been funded by the Federal University of Latin American Integration and the Araucária Foundation.
Reference (open access)
Rivas, A.V.; dos Santos, A.G.V.; de Souza, A.B.; Bueno Junior, G.; de Souza, G.F.; de Souza, E.M.; de Carvalho Nunes, L.; Viana, K.F. Bivalent Vaccine against Streptococcus agalactiae and Aeromonas hydrophila in Nile Tilapia (Oreochromis niloticus): A Laboratory-Phase and Large-Scale Study. Animals 2023, 13, 3338. https://doi.org/10.3390/ani13213338
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.




