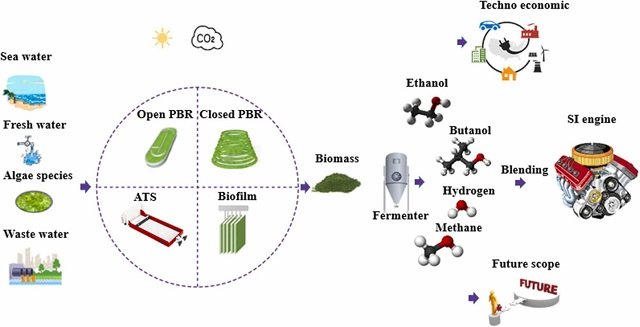
Two consultations on antimicrobial resistance (AMR) risks in aquaculture were jointly organised in Bangkok from 4-7 September by FAO and NACA with much appreciated financial support from FAO and USAID. The consultations were attended by seventeen governments in the Asia-Pacific region, the World Organization for Animal Health (OIE), WorldFish and Chulalongkorn University.
Although control over the use of antimicrobial substances has been strengthened over the past twenty years, mainly from the perspective of international trade and food safety, they are still commonly used in livestock industries and controls over the production and use of antimicrobial substances in aquaculture is still far from adequate or effective. Improper and imprudent use of these substances can significantly contribute to the development of resistance in microorganisms, due to the nature of the aquatic environment and the ways in which cultured animals are handled.
AMR is a growing issue with significant implications for both human and animal health. However, data on pathogen resistance in aquaculture and other livestock industries has not been routinely or systematically collected. The purpose of the regional consultations was to initiate action on this issue, identifying interventions to assess antimicrobial usage in Asian aquaculture and a strategy to minimise the long term AMR risks.
In this context FAO, NACA and USAID are working together to undertake a regional assessment on antimicrobial use (AMU) and the associated risks of AMR in aquaculture. This study will assess the current status of AMU in selected countries as well as their regulation and governance, and identify major issues, gaps and constraints in minimising AMR risks.
The purpose of the regional consultations was to identify actions and develop a strategy to address AMR risks associated with aquaculture, based on an assessment of the status of AMU and AMR. This initiative is part of a broader, coordinated “One Health” movement across the entire human health and agricultural sectors to address prudent usage of antimicrobial substances to reduce AMR risks.
The meeting was opened with welcome remarks from Ms Xiangjun Yao, Regional Programme Leader for FAO-RAP; Dr Daniel Schar, Senior Regional Emerging Infectious Diseases Advisor for USAID; Dr Chumnarn Pongsri, Deputy Director General of the Thai Department of Fisheries; and Dr Cherdsak Virapat, Director General of NACA.
The first consultation addressed the status of AMU and AMR in the region, current national initiatives and regulatory instruments, and the development of a regional framework for AMR surveillance. Issues discussed included:
– The status of antimicrobial usage and antimicrobial resistance in the region.
– Antimicrobial resistance surveillance initiatives in Asian aquaculture.
– Development of a framework for antimicrobial resistance surveillance in Asia.
– A regional overview of current laws and regulations relevant to antimicrobial usage and resistance.
– Antimicrobial resistance in important bacterial diseases of aquaculture.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
The discussions paved the way for the second consultation, which concerned the development of a regional guideline on AMR surveillance in aquaculture. Issues addressed included:
– Developing the framework for antimicrobial resistance monitoring and surveillance in Asia, including harmonisation of national antimicrobial resistance surveillance and monitoring programs for aquatic animals under the OIE Aquatic Animal Health Code; risk analysis of foodborne antimicrobial resistance and the Codex Alimentarius; methods and performance standards on AST from aquatic bacterial isolates and the Assessment Tool for Laboratory and AMR Surveillance Systems (ATLASS).
– Establishing the principles, purpose and objectives of the AMR surveillance guidelines for aquaculture including design, priorities and sampling strategies, methods for bacterial isolation, development of antibiotic panels and isolate storage.
– Guidelines on data management, including tools, storage and sharing of AMR surveillance data and implementation plans.
– The endpoint envisaged for this initiative is the development of a guideline and framework for AMR monitoring and surveillance in Asia that will include regional guidelines on sampling approaches, laboratory testing and data management. These are anticipated to contribute towards the development of evidence-based treatments guidelines for common pathogens in aquatic animals and to reinforce good veterinary practices in lieu of unwarranted metaphylaxis and broad-spectrum preventative treatments.
Source: ENACA
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.