
The turbot (Scophthalmus maximus) is a flatfish with great taste and nutritional value. It inhabits the temperate waters of the Atlantic and the Mediterranean and is prized for its firm, white flesh, rich in proteins and omega-3 fatty acids.
In Europe, the turbot is among the fish species that have best adapted to intensive farming. The first steps in the production of this fish were taken in Scotland (United Kingdom) during the 1970s (Rodríguez 2011), but later turbot aquaculture quickly expanded to Spain and France (Pereiro et al., 2016). Currently, marine turbot aquaculture has been developed in fish farms mainly located in Spain, Portugal, and China; however, turbot marine farms can also be found in Bulgaria, Canada, France, Romania, Turkey, South Korea, Chile, and Norway.
In this article, we will explore the many aspects of turbot aquaculture. We also provide information about its biology, nutritional value, and culinary uses.
Biology of the Turbot
Taxonomy of the Turbot
- Kingdom: Animalia
- Phylum: Chordata
- Class: Actinopterygii
- Order: Pleuronectiformes
- Family: Scophthalmidae
- Genus: Scophthalmus
- Species: Scophthalmus maximus, formerly Psetta maxima (Linnaeus, 1758)
- Name in Spanish: Rodaballo
- Name in Galician: Rodaballo
- Name in Catalan: Remol empetxinat, turbó
- Name in Basque: Erreboilo arrunta, errebolu
- Name in English: Turbot, brat, breet, britt, or butt
- Name in French: Turbot
- Name in Dutch: Tarbot
- Name in Italian: Rombo grande
- Name in German: Steinbutt
- Name in Portuguese: Pregado, rodavalho
- Name in Turkish: Civili Kalkan
Distribution and Habitat
It is distributed along the coasts of the Black Sea and the Mediterranean Sea, as well as in the northeast and east of the Atlantic Ocean.
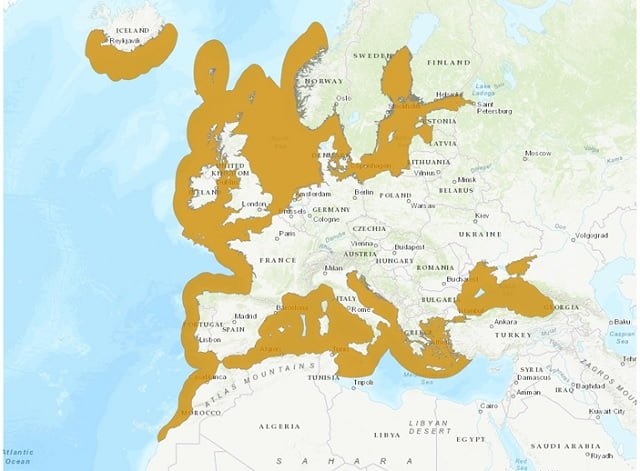
The turbot is a benthic fish, typical of cold to temperate waters. In their larval stage, they remain floating, but once they complete metamorphosis, they stay at the bottom. In the juvenile stage, turbot already have benthic habits, meaning they remain settled on the bottom, hidden while waiting for prey to approach. During this developmental phase, they are found in coastal areas and gradually move away from the coastline, further into the sea as they grow. They prefer sandy or muddy bottoms at depths of up to 150 meters (Martínez et al., 2016).
Morphology
- Flattened and oval-shaped body
- Pigmented ocular side (brown or green) and blind side white
- The eyes are located on the left side of the body
- Large mouth with prominent teeth
- Two dorsal fins, one anal fin, and two pectoral fins
- Forked caudal fin.

Nutritional properties of turbot
Nutritional value
Turbot meat is white and highly prized, known for its low-fat content, making it recommended for weight loss diets, cholesterol issues, and for people with diabetes.
Table: Nutritional value per 100 grams of turbot (Scophthalmus maximus).
| Nutrient | Amount |
|---|---|
| Dry matter | 20.9 grams |
| Protein | 15.9 grams |
| Fat | 2.4 grams |
| Glycogen | 0.1 grams |
| Vitamin A | 4 µg |
| Vitamin D | 1.7 µg |
| Vitamin E | 0.6 µg |
| Saturated fatty acids | 23.0 mg |
| Monounsaturated fatty acids | 31.9 mg |
| Cholesterol | 54 mg |
| Calcium | 16 mg |
| Iron | 0.2 mg |
| Potassium | 290 mg |
| Phosphorus | 160 mg |
Health Benefits
- Reduces the risk of cardiovascular diseases
- Improves brain function
- Protects eye health
- Strengthens the immune system
Potential Health Risks (mercury)
Turbot may contain low levels of mercury, so it is recommended not to consume it in large quantities, especially for pregnant and lactating women.
Wild or Farmed Turbot?
This is a difficult question to answer as it depends on personal preferences. However, we present the table below summarizing the key differences between wild turbot and farmed turbot, providing useful information for making informed decisions about the choice of turbot type based on your preferences and individual considerations.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
Table: Comparison between wild turbot and farmed turbot.
| Characteristic | Wild | Farmed |
|---|---|---|
| Taste and Texture | Pronounced, firm texture | Consistent, controlled texture |
| Diet | Natural marine habitat | Controlled in farms |
| Availability | Seasonal, subject to seasons and regulations | Available year-round |
| Quality Control | Less controlled | Farm monitoring |
| Sustainability | Variable depending on fishing practices | Sustainable practices possible |
| Cost | Potentially higher | Potentially lower |
Comparison of Turbot vs. flounder
A common question asked by specialists, and particularly consumers, is the differences between turbot and sole. Below, I present a table with the main differences:
Comparison table of turbot and sole.
| Characteristic | Turbot | Flounder |
|---|---|---|
| Scientific Name | Scophthalmus maximus | Pleuronectes platessa |
| Size | Can reach up to 1 meter in length and weigh up to 20 kg. | Generally does not exceed 50 cm in length and 2 kg in weight. |
| Body Shape | Oval and flattened. | Oval and flattened, but more elongated than turbot. |
| Color | Brown or green ocular side with dark spots. Blind side white. | Brown or green ocular side with dark spots. Blind side white. |
| Eyes | Small and located on the left side of the body. | Small and located on the right side of the body. |
| Feeding | Carnivorous. Feeds on small fish, crustaceans, and other invertebrates. | Carnivorous. Feeds on small fish, worms, and other invertebrates. |
| Habitat | Sandy or muddy bottoms at depths of up to 150 meters. | Sandy or muddy bottoms at depths of up to 200 meters. |
| Distribution | Northeastern Atlantic and Mediterranean. | North Atlantic, North Pacific, and Baltic Sea. |
| Commercial Value | Important species in aquaculture. Considered a high-quality fish. | Species of lower commercial value than turbot. |
| Taste | Firm and white flesh with a delicate flavor. | Firm and white flesh with a slightly stronger flavor than turbot. |
Feeding
Turbot exhibits carnivorous habits. Turbot fry begin their benthic life by feeding on small crustaceans. In the juvenile stage, they feed on small fish, mollusks, and crustaceans, while adult turbot prey on teleost fish and cephalopods, with their feeding behavior primarily nocturnal (Menú & Person, 1991).
Currently, commercial feeds are available to feed turbot at different stages of growth. Furthermore, a series of strategies are being implemented to reduce the use of fishmeal in their diet. Hoerterer et al., (2022) highlighted that fish by-products are suitable substitutes for commercial fishmeal and that protein sources derived from plants or land animals can replace up to 20% of the total fish-derived ingredients without compromising the growth performance and body composition of juvenile turbot.
Lipids
Ma et al., (2022) evaluated the effects of different levels of lipids in the diet on the intestinal physiology of juvenile turbot and found that a 12% lipid level in the diet increased the activities of intestinal digestive enzymes and maintained the stability of turbot intestinal microflora. Meanwhile, Wencong et al., (2022) determined the following lipid levels in fish feed according to three growth stages:
| Size | Lipid Level |
|---|---|
| Small (9.13 g) | 130.1 g/kg |
| Medium (50 g) | 120.1 g/kg |
| Large (80 g) | 107.7 g/kg |
Vitamins
Studies have shown clear evidence that vitamin D3 (VD3) positively regulates innate and adaptive immunity in fish, which is beneficial for fish defense against pathogen infection such as Edwardsiella tarda (Liu et al., 2022).
Shao et al., (2022) determined that the optimal level of vitamin D3 (VD3) in turbot feed is around 400 μg/kg, while deficiency or overdose of VD3 in diets induced intestinal inflammation, reduced intestinal microbiota diversity, and affected host resistance to bacteria.
Immunostimulants
The use of immunostimulants in feeding is an effective way to protect health and promote the growth of different aquaculture species. Sun et al., (2020) reported that astragalus polysaccharides (APS) can be used at a rate of 150 mg/kg to significantly improve growth performance, antioxidant activity, and maintain an active immune response in turbot.
Wijerath et al., (2020) determined that the inclusion of 1.2% taurine in juvenile turbot diets allows for significantly greater hyperplasia fiber growth and textural properties in fish muscle.
Reproduction
Turbot is a dioecious species, and they reproduce naturally once a year, in spring (between February and April in the Mediterranean and from May to July in the Atlantic), gathering in chosen spawning areas, at depths of about 30 meters (Medas, 2004).
Female turbot mature sexually from 2 years old when they are 40 cm long and weigh 1 kg, while males can fertilize from 1.3 kg in weight and when they reach 30 cm (3 years old). The female swims and releases the eggs simultaneously, while one or more males follow releasing sperm to come into contact with the suspended eggs in the water. Females spawn approximately 3 million eggs, 1mm in diameter and transparent. Fertilized eggs are pelagic, incubation typically lasts for a week (varies with temperature), at which point they hatch and the larvae emerge (Saavedra, 1998).
Breeder Management
The first step of the production process is obtaining quality breeding adults that ensure a good quantity and quality of fertilized eggs.
Recently, FAO published a manual describing turbot breeding practices in the Black Sea (Scophthalmus maximus); the document details the production of live feed for turbot larvae starting with microalgae, followed by rotifers and Artemia production; breeder management; procedures for optimal larval and juvenile rearing in the fourth section; and presents recent applications of biotechnologies to turbot production, namely cryopreservation, triploidy, and exclusive female production.
Turbot Farming
Growth
The growth rate of male and female turbot is relatively equal until they reach a length of approximately 45 cm at the age of 6 to 7 years, after which the growth of males slows down more than that of females. Females typically measure between 50 and 80 cm in length, although they have reached sizes of 90 cm at 20 years of age, while males typically measure 45 cm in length, reaching sizes of 60 cm at 15 years old that they can live up to (Subpesca, 2004).
In aquaculture, turbot has rapid growth. It can reach a market weight of 1 kg in 18 months.
Water Quality Parameters
Table: Water Quality Parameters for Turbot Aquaculture.
| Parameter | Range |
|---|---|
| Temperature | 8 to 22 ºC, optimum: 16 ºC |
| Dissolved Oxygen | 4 to 8 mg/L, Hypoxia threshold: 2 mg/L (30% saturation) |
| Salinity | 25 to 35 ‰, optimal value: 30 ‰ |
| pH | 7.9 – 8.2 |
| Ammonia | Optimal: 0.3 to 0.5 mg/L, Lethal: > 5 mg/L |
| Nitrate (NO3-N) | 50 mg/L |
| Photoperiod | 12 hours light: 12 hours darkness |
| Light Spectrum in Tank | Blue (450 nm) |
Cultivation Process
There are few facilities where the entire turbot acquisition process is carried out since the hatchery phase (larvae and fingerlings acquisition and breeding) differs significantly from pre-fattening and fattening, so it is usually carried out in specialized facilities, called hatcheries, which supply fingerlings to pre-fattening farms and these to fattening farms. Despite this difficulty, some companies have chosen to carry out the entire process, without depending on external supplying companies, to have greater control over the quality of the fingerlings, which is key to the farm’s profitability. Only companies with large production undertake the entire process, which allows them to make the hatchery investment profitable (Saavedra, 1998).
Hatchery
In this phase, fingerlings weighing between 2 and 10 grams are obtained from breeding adults. It is the most delicate phase of the production process in which the highest mortality will occur, since turbot eggs, larvae, and fingerlings are handled, which are the most sensitive stages to variations and physicochemical imbalances. The good condition and development of the fingerlings are fundamental for the performance of the pre-fattening and fattening phases.
Pre-fattening
Pre-fattening is the intermediate phase of the production process between breeding and fattening. Pre-fattening involves the development of fish from when turbot weighs between 1 and 10 grams until they reach 100 or 150 g; this phase lasts approximately between 7 and 9 months. The weight at the end of the process is determined by the preferences imposed by the fattening farm to which the batches are destined and depends on each farm’s policy.
The stocking density decreases from 1,000 to 500 ind.m-2 between the third and sixth month, maintaining an average load of 15 kg.m-2 at the end of the pre-fattening with a water flow greater than 0.02 m3/kg/h.
Pre-fattening is carried out in facilities located on land, near the coast. The main element of these facilities is the culture tanks, which are self-cleaning square or circular tanks with central drainage, of shallow depth (40 to 50 cm).
Once the pre-fattening process is completed, the turbots are transported to fattening farms, which will fatten the fish to the commercial weight for the market (Medas, 2004).
Fattening
Fattening covers the period from when the fish weigh between 100 and 150 g, which arrive at the installation, until they reach the commercial size, with an approximate weight of 2 kg; this weight varies according to market commercial needs and installation biomass management policy. The fattening process lasts approximately between 17 and 22 months.
In fattening, more cultivation area is required, and the culture tanks are larger to better utilize the farm space, taking advantage of the fact that turbot, due to their size, are less sensitive to variations in some parameters and withstand less controlled situations.
The flow rate will be higher than that used in pre-fattening, the intake, distribution, drainage, treatment, etc., facilities must be larger and with new safety measures (Medas, 2004). Li et al., (2019) suggest a speed of approximately 0.9 bl s-1 (body length per second) to promote growth and obtain better innate immunity of turbot cultivated in aquaculture recirculation systems.
One of the problems encountered in fattening is when turbot reaches maturity. From that moment on, turbot ceases to grow for an approximate period of four months. This cessation of growth has important economic implications. To prevent turbot from reaching maturity, the photoperiod is controlled, and the sales size is reduced. The stocking densities in the fattening phase are 12 – 15, 15 – 25, and 25 – 35 kg.m-2 (Fundación Chile, 2004).
Tank Cultivation
Up to this point, due to turbot’s benthic behavior, only tank cultivation on land (20 to 100m3) with highly controlled cultivation conditions has been considered; circular self-cleaning tanks or raceways fed by pumping, are covered to prevent sunlight damage to the fish’s skin.
Cultivation is carried out with individual densities between 20 and 40 kg.m-2; it is very important throughout the stage to classify the fish by size since hierarchies are formed in the tanks by size.
Currently, maintaining batches of large-sized turbot in a land installation is very expensive since water requirements are very high, and large surfaces are required.
Cage Cultivation
Cage cultivation aims to reduce production costs. The bottom of the cage must be rigid enough to be used by turbot. It must allow water to pass through and be flexible; sheltered areas are needed where water renewal is constant; they are kept at a height of 1 to 2m, to avoid too much light on the cage bottom, they are covered with dark tarpaulins (Saavedra, 1998).
The crucial element of turbot cages is to achieve a suitable bottom. In trials, the growth rate, feed performance, and conversion factors in cages with rigid, flexible bottoms, and in tanks are compared (TecnoPress, 2003).
Harvesting
Harvesting is an operation of collecting or capturing fish from the tank and floating cages where they remain. For this, nets with handles are used, which are handled by the operating personnel responsible for capturing the turbots. Capturing is not complicated since they are not very lively fish; nevertheless, to facilitate this task, half of the tank water can be drained so that they have less water volume to escape.
Once captured, they are deposited, according to the purpose of the capture, either in ice tanks for slaughter or in special trays to be classified by size and subsequently distributed. In the latter case, when captured to be reclassified by size, the capture operation must be carried out quickly so as not to keep the fish in the tank with little water and little oxygen, since due to the fish’s agitation, oxygen consumption will be higher than usual.
Diseases
Turbot, like all living beings, is susceptible to bacterial, viral, and parasitic diseases. In Rodriguez’s manual (2011), you can find more detailed information on the diseases that affect turbot raised in fish farms.
VHSV is one of the main pathogens that affect turbot. Fish affected by this virus show lethargy, darkening of the skin, exophthalmia, anemia (pale gills), hemorrhages at the base of the fins, gills, mouth, eyes, and skin, distended abdomen due to edema in the peritoneal cavity, and severe abnormal swimming behavior.
Pereiro et al., (2016) reported that certain molecules can be selected as possible antiviral treatments due to their high protective effect against VHSV, and the use of resistance markers for selective breeding is one of the most attractive approaches.
Conclusion
Turbot is a flat fish with great taste and nutritional value. Its aquaculture breeding allows meeting market demand, but it is important to do it sustainably to minimize environmental impact. Turbot is a healthy food that can be prepared in various ways and is an excellent choice for a balanced diet.
References
Aydın, I., Küçük, E., Polat, H., Haşimoğlu, A. & Altuntaş, A. 2023. Black Sea turbot – A comprehensive production manual. FAO Fisheries and Aquaculture Technical Paper, No. 693. FAO, Rome. https://doi.org/10.4060/cc6224en
Cardinale, M., Chanet, B., Martínez Portela, P., Munroe, T.A., Nimmegeers, S., Shlyakhov, V., Turan, C. & Vansteenbrugge, L. 2021. Scophthalmus maximus. The IUCN Red List of Threatened Species 2021: e.T198731A144939322. https://dx.doi.org/10.2305/IUCN.UK.2021-2.RLTS.T198731A144939322.en
Hoerterer, C., Petereit, J., Lannig, G. et al. Sustainable fish feeds: potential of emerging protein sources in diets for juvenile turbot (Scophthalmus maximus) in RAS. Aquacult Int 30, 1481–1504 (2022). https://doi.org/10.1007/s10499-022-00859-x
Jia, Y., Wang, J., Gao, Y., & Huang, B. (2021). Hypoxia tolerance, hematological, and biochemical response in juvenile turbot (Scophthalmus maximus. L). Aquaculture, 535, 736380. https://doi.org/10.1016/j.aquaculture.2021.736380
Li, X., Ji, L., Wu, L., Gao, X., Li, X., Li, J., & Liu, Y. (2019). Effect of flow velocity on the growth, stress and immune responses of turbot (Scophthalmus maximus) in recirculating aquaculture systems. Fish & Shellfish Immunology, 86, 1169-1176. https://doi.org/10.1016/j.fsi.2018.12.066
Liu, J., Shao, R., Lan, Y., Liao, X., Zhang, J., Mai, K., Ai, Q., & Wan, M. (2021). Vitamin D3 protects turbot (Scophthalmus maximus L.) from bacterial infection. Fish & Shellfish Immunology, 118, 25-33. https://doi.org/10.1016/j.fsi.2021.08.024
Ma, X., Bi, Q., Kong, Y., Xu, H., Liang, M., Mai, K., & Zhang, Y. (2022). Dietary lipid levels affected antioxidative status, inflammation response, apoptosis and microbial community in the intestine of juvenile turbot (Scophthalmus maximus L.). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 264, 111118. https://doi.org/10.1016/j.cbpa.2021.111118
Martínez, P., Robledo, D., Rodríguez-Ramilo, S. T., Hermida, M., Taboada, X., Pereiro, P., Rubiolo, J. A., Ribas, L., Gómez-Tato, A., Álvarez-Dios, J. A., Piferrer, F., Novoa, B., Figueras, A., Pardo, B. G., Fernández, J., Viñas, A., & Bouza, C. (2016). Turbot (Scophthalmus maximus) genomic resources: Application for boosting aquaculture production. Genomics in Aquaculture, 131-163. https://doi.org/10.1016/B978-0-12-801418-9.00006-8
MEDAS.2004. MANUAL DE PRODUCCION DEL RODABALLO. PROYECTO MEDAS 21. Medidas Contra La Exclusión Y El Desempleo En Áreas Litorales. Editorial EQUAL. TOMO I. Universidad Politécnica de Madrid. 43p.
MENU, B. & J. PERSON .1991. “Fase de Criadero y Engorde” del Capítulo El cultivo de Peces planos: Lenguado, rodaballo. En: ACUICULTURE, Gilbert Barnabé Vol. II. Edic. Omega S.A. Barcelona – España. pp: 625-642.
Pereiro, P., Figueras, A., & Novoa, B. (2016). Turbot (Scophthalmus maximus) vs. VHSV (viral hemorrhagic septicemia virus): a review. Frontiers in Physiology, 7, 198611.
Rodríguez José. 2011. Cultivo del Rodaballo (Scophthalmus maximus). Cuadernos de Acuicultura No 4. Fundación Observatorio Español de Acuicultura. 46 p.
SAAVEDRA H. 1998 “Cultivo del Turbot”. Monografía para optar el Título Profesional de Ingeniero Pesquero Oceanógrafo e Hidrobiologo, UNIVERSIDAD NACIONAL FEDERICO VILLARREAL. 61P.
Shao R, Liu J, Lan Y, et al. Vitamin D impacts on the intestinal health, immune status and metabolism in turbot (Scophthalmus maximus L.). British Journal of Nutrition. 2022;128(11):2083-2096. doi:10.1017/S0007114522000125
Sun, Y., Wang, X., Zhou, H., Mai, K., & He, G. (2020). Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish & Shellfish Immunology, 99, 603-608. https://doi.org/10.1016/j.fsi.2020.02.056
Wencong Zhang, Zhijie Dan, Yanwen Zhuang, Jichang Zheng, Ye Gong, Yongtao Liu, Kangsen Mai, Qinghui Ai, “Effects of Dietary Lipid Levels on Growth, Digestive Enzyme Activities, Antioxidant Capacity, and Lipid Metabolism in Turbot (Scophthalmus maximus L.) at Three Different Stages“, Aquaculture Nutrition, vol. 2022, Article ID 1042263, 18 pages, 2022. https://doi.org/10.1155/2022/1042263
Wijerath Wiriduge, H. A. S., Zhang, Y., Liu, J., Yang, M., Zhang, W., & Mai, K. (2020). Dietary taurine improves muscle growth and texture characteristics in juvenile turbot (Scophthalmus maximus). Aquaculture Reports, 17, 100305. https://doi.org/10.1016/j.aqrep.2020.100305
Wu, L., Wang, Y., Li, J., Song, Z., Xu, S., Song, C., Han, M., Zhao, H., Zhou, L., Wang, Y., Li, X., & Yue, X. (2021). Influence of light spectra on the performance of juvenile turbot (Scophthalmus maximus). Aquaculture, 533, 736191. https://doi.org/10.1016/j.aquaculture.2020.736191
Yu, J., Wang, Y., Xiao, Y., Li, X., Zhou, L., Wang, Y., Du, T., Ma, X., & Li, J. (2021). Investigating the effect of nitrate on juvenile turbot (Scophthalmus maximus) growth performance, health status, and endocrine function in marine recirculation aquaculture systems. Ecotoxicology and Environmental Safety, 208, 111617. https://doi.org/10.1016/j.ecoenv.2020.111617
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.







