The grass carp (Ctenopharyngodon idella) is the most widely cultured freshwater fish in the world, with a total production of 5.87 million tons reported in 2023. In China, it represents the species with the highest production among farmed fish. However, the sector faces a critical temporal challenge: traditional breeding methods can take between 15 and 20 years to produce new varieties because the sexual maturation period of the species is approximately 5 years.
To break this bottleneck, a research team from the Key Laboratory of Freshwater Fish Reproduction and Development (Ministry of Education) and the College of Fisheries at Southwest University in Chongqing, China, has utilized CRISPR/Cas9 technology to disrupt the myostatin gene, achieving results that could reduce the time required to obtain improved varieties to only 5 years. The findings have been published in the scientific journal Reproduction and Breeding.
Key Study Findings
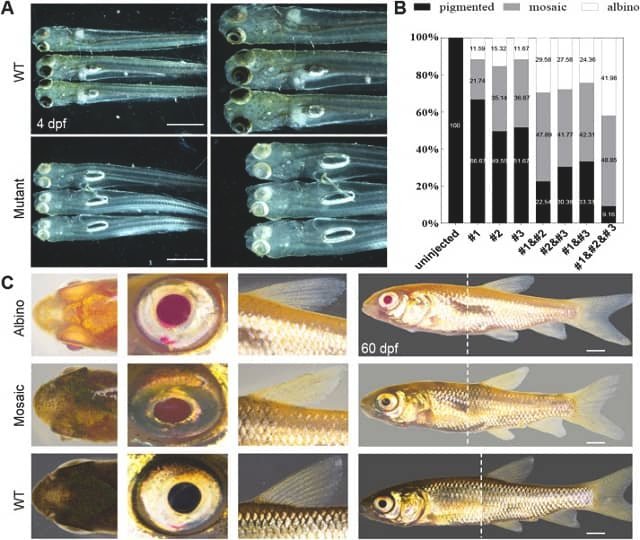
- Editing Efficiency: Approximately 85% of the injected individuals carried mutations at the target site of the mstnb gene.
- Biomass Increment: Mutants showed an average increase of 18% in body weight and 14.5% in length at four months of age.
- Hyperplastic Growth: The greater weight is due to an increase in the total number of muscle fibers, rather than their individual thickness.
- Genetic Activation: The editing activated key genes such as myod1 and vegfba, which are essential for muscle differentiation and proliferation.
The “Molecular Brake” of Muscle Growth
Myostatin (mstn) is known in science as a negative regulator of muscle fiber growth in vertebrates. In grass carp, researchers identified two variants: mstna and mstnb. After tissue analysis, it was discovered that only the mstnb gene is expressed in skeletal muscle, which made it the primary target for improving growth.
“The mstnb gene acts as a key molecular brake in the formation of muscles in grass carp,” explained Dr. Shengfei Dai, corresponding author of the study. “By removing this brake, we were able to unlock the fish’s natural capacity to produce more muscle cells and grow faster.” This genetic disruption allows the fish’s organism not to limit its muscular development in the way it naturally would, facilitating more efficient weight gain in the juvenile stages.
Methodology: Precision with CRISPR/Cas9
The process consisted of the co-injection of the Cas9 protein and guide RNA (sgRNA) into embryos at the single-cell stage. The target was a 20 bp sequence located in the first exon of the mstnb gene.
- Mutation Induction: Various frameshift mutations were generated, which produce non-functional proteins.
- Validation: The success of the editing was confirmed through T7 endonuclease (T7E1) assays, heteroduplex mobility assay (HMA), and Sanger sequencing.
- Molecular Results: The expression of the mstnb gene was significantly reduced in the mutants, while the mRNA levels of pro-growth genes such as myod1 (myogenic regulatory factor) increased.
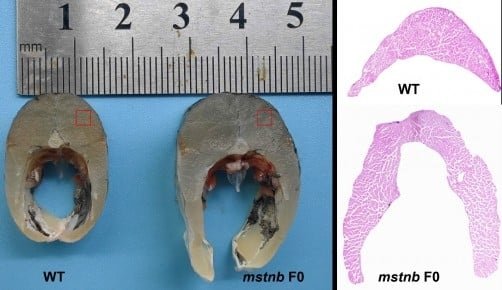
A Surprising Finding: More Fibers, Not Thicker Ones
One of the most revealing points of the study emerged when analyzing the microstructure of the muscle. In many species, myostatin editing produces hypertrophy (larger fibers). However, the grass carp responded differently. Through histological examinations with Hematoxylin and Eosin (H&E) staining, it was observed that there was no significant difference in the thickness of individual fibers between wild-type and edited fish. Nonetheless, the total number of fibers in the same anatomical location was significantly higher in the mutants.
“A surprising result was that the muscle fibers themselves did not become much larger; instead, the fish built more fibers,” noted Dr. Dai. “We expected the fibers to be thicker, but what we actually saw was a dramatic increase in the number of fibers. This suggests that different growth pathways can be selectively influenced through genetic editing.” This type of growth, termed muscle hyperplasia, is of great interest to the industry, as it is often associated with a firm muscle texture and high meat quality in aquaculture species.
Comparison and Potential in Aquaculture
Although the 18% increase in weight is a notable advancement, the study acknowledges that other species have shown more drastic responses, such as the 49.5% increase observed in Nile tilapia. This highlights the differences between species in the regulation of mstn-mediated myogenesis. The development of mutant grass carp offers a new source of genetic material to improve growth traits. Furthermore, the combination of this technology with gynogenesis techniques could drastically accelerate the supply of new varieties to the market.
Conclusion
The study concludes that the disruption of the mstnb gene is an effective strategy to enhance growth in grass carp. By acting on the proliferation of muscle fibers, a significant increase in marketable biomass is achieved, laying the foundations for a more efficient and productive precision aquaculture. This work was funded by the Agriculture Biobreeding Major Project (2023ZD0405503) and the Fundamental Research Funds for the Central Universities (SWU-XDJH202311 and SWU-KQ22061).
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
Contact
Shengfei Dai
College of Fisheries, Southwest University, Chongqing, 400715, China.
Email: dsf20216036@swu.edu.cn
Reference (open access)
Zhao, P., Shen, Y., Cheng, J., Zhang, L., Qu, Z., Li, W., Liu, X., Li, M., & Dai, S. (2025). Generation of fast-growth grass carp by mutation of mstnb via CRISPR/Cas9 system. Reproduction and Breeding, 5(4), 163-170. https://doi.org/10.1016/j.repbre.2025.09.001
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.