In recent years, sea lice infestations have caused concerns, not only for their impact on the marine aquaculture industry (especially salmon farming), but also for reported attacks on swimmers at beaches in Australia.
This report aims to provide information on the varieties of sea lice that affect the marine fish farming industry, particularly salmon farming, as well as identify some control measures and treatment methods being employed against sea lice worldwide.
What is a sea lice?
A sea louse is a parasitic copepod, also known as a marine louse, belonging to the family Caligidae (Nagasawa 2004); this family has 30 genera and 509 species.
The most important genera are Lepeophtherius, Caligus, and Pseudocaligus, as they cause high mortalities in the aquaculture industry and have, in some cases, been responsible for infestations on bathers.
Hemmingsen et al., (2020) published a review of the geographical distribution and host preferences of six species of sea lice that affect the salmon farming industry worldwide: C. elongatus, C. curtus, C. clemensi, C. rogercresseyi, C. teres, and C. orientalis.

Understanding sea lice
Sea lice develop quickly in temperate temperatures, with one generation completed in almost a month at a temperature of 15 oC, producing a large number of eggs, leading to rapid population growth and consequently infestation.
Sea lice affect salmon and all marine fish in several ways: loss of scales, which makes the fish susceptible to other diseases (O’Donohoe et al., 2004), and damage to the fish, which reduces the product’s quality. These parasites feed on the skin, mucus, and blood of the fish.
The injuries caused by sea lice lead to osmoregulatory imbalances and fungal and bacterial infections.
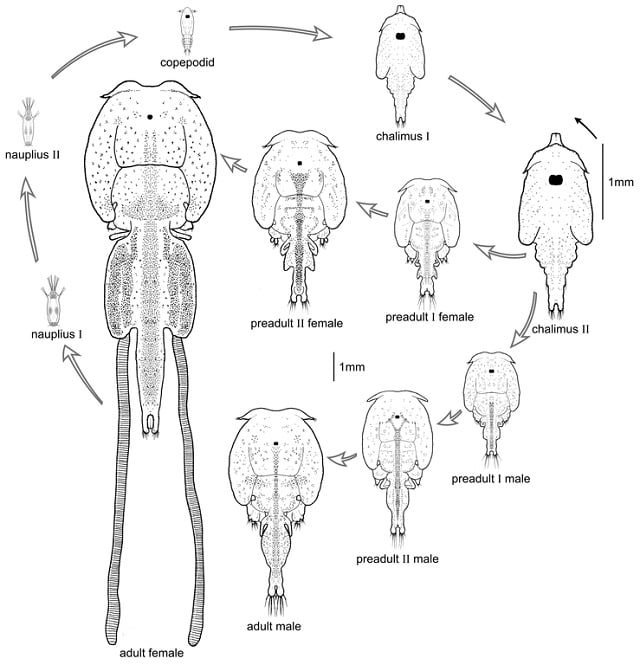
Sea lice commonly have 10 developmental stages. These include two free-living naupliar stages, one free-swimming copepodid infection stage, four chalimus stages that attach to the fish, two pre-adult stages, and an adult stage. Pre-adult and adult stages roam freely on the host’s surface.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
Impacts of sea lice on the marine aquaculture industry
One of the main pests affecting the marine fish farming industry is sea lice. Ectoparasitic copepods are the main pathogens of salmon farms in Norway, Scotland, Ireland, Canada, and Chile.
Abolofia et al. (2017) highlight that sea lice generate losses of biomass per cycle in salmon farming of 3.62 to 16.55%, and that the average cost of damage caused by sea lice is US$ 0.46 per kilogram of harvested biomass.
These parasites generate significant losses to aquaculture producers, as the growth rate of farmed fish, their quality, and the increase in production costs due to parasite treatment expenses are reduced.
On the other hand, the use of antiparasitic chemicals could have negative impacts on aquatic species and the ecosystem surrounding marine fish farms. Regarding this, Parson et al., (2020) suggest that deltamethrin represents a significant risk to European lobster larvae (Homarus gammarus) in the Norwegian marine environment, while the impact of azamethiphos may be less severe.
Distribution of sea lice
In particular, Lepeophtheirus salmonis and Caligus elongatus are important pathogens in Europe and North America (Nagasawa 2004). However, species of the genus Caligus have been reported worldwide; it should be noted that problems with sea lice infestations are more significant in countries where marine aquaculture is intensively developed.
Lepeophtheirus salmonis
This species has been found in salmon in Ireland (O’Donohoe et al, 2004). According to Nagasawa (2004), L. salmonis is a common parasite of chum salmon (Oncorhynchus keta) and pink salmon (O. gorbuscha) in the waters north of Japan; this parasite is also found in salmonids in Korea and Russia. Nagasawa (2004) also reports that these parasite species are present in coho salmon (O. kisutch) and rainbow trout (O. mykiss) raised in coastal waters north of Japan, but infection is not a serious problem because young fish are raised and harvested in less than a year, so the fish are not cultured during the summer.
On the other hand, in countries such as Scotland, Ireland, Norway, and Canada, L. salmonis causes serious damage to Atlantic salmon farms (Nagasawa 2004).
Caligus
O’Donohoe et al (2004) report the presence of C. elongatus in salmonid farms in Ireland and indicate that it infects more than 80 marine fish. In this regard, Robaldo et al (2002) suggest a high potential for infestation of juvenile flounder (P. orbignyanus) by Caligus sp. in Brazil.
Species of sea lice in the genus Caligus cause the greatest problems in Southeast Asia. C. patulus infests the skin and gills of milkfish (Chanos chanos) in brackish water ponds in the Philippines and Indonesia.
Within this group, Caligus orientalis is a parasite of marine and brackish water fish in Japan and neighboring countries (Taiwan, China, Korea, and Russia); this parasite has been reported in more than 20 species of fish from different orders and families. C. orientalis has infested fish such as rainbow trout in Japan, lisa (Mugil cephalus) and black porgy (Acanthopagrus schlegeli) in Taiwan, and Mozambique tilapia (Oreochromis mossambicus) in China (Nagasawa 2004).
Prevention measures
Research on prevention methods for sea lice infestations in aquaculture has not received much attention from researchers. Preventive methods emphasize the host’s resistance traits while reducing encounters between the host and the parasite (Barrett et al., 2020).
Barrett et al. (2020) summarized a range of potential and existing preventive methods, among which the barrier technology stands out with a 76% reduction in infestations, as well as spatiotemporal geographic management, manipulation of swimming depth, functional foods, repellents, and masking host signals, which may generate smaller reductions that can be additive when used in combination with barrier technologies.
On the other hand, Coates et al., (2023) developed a model for the metapopulation and evolutionary dynamics of the salmon louse (Lepeophtheirus salmonis), and demonstrated that evolutionary models can produce quantitative predictions at large spatial and temporal scales and for a variety of pest control scenarios.
Nutrition
Leclercq et al. (2020) studied the supplementation of salmon’s diet with a mannan-rich yeast fraction, reporting an increase in skin mucus and the density of goblet cells. This allowed for a 16.6% reduction in susceptibility to an acute challenge of standard copepods, along with an earlier increase in skin lysozyme activity, widely used as an index of innate immunity.
Barrier technologies
Nilsen et al. (2017) documented the protective effects of closed floating farming systems to reduce infestations of Lepeoptheirus salmonis and Caligus elongatus in Atlantic salmon farming, without adverse effects on fish growth and mortality.
On the other hand, Oldham (2023) reports that the protective efficacy of snorkel barriers was halved under suboptimal salinity and temperature conditions, and that the condition and growth of gills can be negatively affected by snorkel barriers, particularly when there is a halocline. However, it also highlights that the combined use of skirt barriers and behavioral modification reduces lice infestations by over 50% without negative impacts on gill growth or condition.
Vaccines
Vaccines have emerged as the most potentially effective prevention method for sea lice in the aquaculture industry. Casuso et al. (2022) evaluated the use of three vaccine prototypes to combat sea lice and concluded that vaccinated fish showed modulation changes in biological processes such as biological regulation, cellular metabolic processes, and energy production, which may be fundamental for the early stages of C. rogercresseyi fish response.
Treatment for sea lice
A recent study by Aldrin et al. (2023) evaluated the effectiveness of 10 treatments against sea lice, and they concluded that thermal, mechanical, and freshwater treatments kill 70.80% of the lice. They also estimate that feeding treatments with emamectin benzoate kill around 35% of the lice, while hydrogen peroxide bath treatments kill approximately 74%, and pyrethroids kill 50% of the lice. They highlight that the recently approved bath treatment, imidacloprid, kills over 98% of the lice.
The following are the approaches being pursued to combat sea lice:
Genetic selection
Barrett et al. (2020) highlights that the continuous development of sea lice-resistant salmon lineages can lead to long-term improvements if genetic gain is maintained.
Similarly, Robledo et al. (2019) described quantitative trait loci affecting resistance to sea lice (Caligus rogercresseyi) in Atlantic salmon, explaining between 7% and 13% of the heritability of this trait. In the same vein, Cáceres et al. (2021) identified candidate genes in Atlantic salmon and rainbow trout (Oncorhynchus mykiss) linked to sea lice resistance.
Gallardo et al. (2019) emphasize genomic knowledge about hosts and sea lice, with a focus on Salmo salar and Oncorhynchus kisutch as host fish species and Caligus rogercresseyi as the main threat affecting the Chilean salmon industry.
Bioacoustic methods
Solé et al. (2021) studied sea lice’s sensitivity to low-frequency sounds and reported that sea lice’s copepod and chalimus stages were affected by sound exposure. However, further studies are required for this method to be commercially viable.
Chemical control
This method has been the most investigated and consequently the most developed; however, the growing trend is to reduce the use of chemicals to control sea lice due to their negative environmental impacts on aquatic species and marine ecosystem health.
Hannisdal et al. (2020) reported the use of emamectin, cypermethrin, diflubenzuron, and teflubenzuron in the Norwegian salmon industry, and the use of hydrogen peroxide is also known.
Biological control of sea lice
The use of cleaner fish to control sea lice infestations in Atlantic salmon farms is widespread and considered a favorable alternative to current lice removal treatments for the well-being of salmon (Overton et al., 2020). In this regard, the Norwegian salmon industry has adopted the use of cleaner fish for the biological control of sea lice (Barrett et al., 2020b).
Powell et al. (2018) highlight the use of lumpfish (Cyclopterus lumpus) to eat sea lice on farmed salmon and describe the challenges it still needs to overcome to become a viable option for the salmon industry. Similarly, Brooker et al. (2018) describe the farming of ballan wrasse (Labrus bergylta) and lumpfish (C. lumpus).
Currently, it is estimated that approximately 50 million cleaner fish are used only in the Norwegian salmon industry (Barrett et al., 2020b). However, Overton et al. (2020) describe that reported efficacies in the use of cleaner fish allow for reductions of 28% to 100% in the number of sea lice.
Regarding this, Gentry et al. (2020) emphasize that the efficiency of using cleaner fish in commercial Atlantic salmon farming cages depends mainly on the prevention strategies used.
Challenges and perspectives
Therapeutic chemical methods are the most commonly used to control sea lice infestations.
On the other hand, little attention has been paid to genetic selection as a method of sea lice control, even though it could be an interesting method if combined with adequate prevention measures.
Controlling infestations of sea lice will depend on the combined use of preventive measures and chemical methods. However, attention must be paid to the potential resistance that sea lice may generate to chemical treatment.
Effective measures to control sea lice should be explored, as this organism has the ability to parasitize different fish species and thus also poses a threat to any future marine fish farming.
Conclusion
Sea lice are one of the main threats to the salmon industry worldwide. However, significant advances have been made in prevention and control methods.
Among the prevention measures, nutrition, barrier technologies, and vaccines show the greatest potential. For its part, treatments such as genetics, the use of cleaner fish, or acoustic methods are the most promising.
Studies on the biology, prevention, and treatment of sea lice have intensified, and models are now available to study them.
References
Abolofia, J., Asche, F., & Wilen, J. E. (2017). The cost of lice: quantifying the impacts of parasitic sea lice on farmed salmon. Marine Resource Economics, 32(3), 329-349.
Aldrin M., R.B. Huseby, L.C. Stige, K.O. Helgesen. 2023. Estimated effectiveness of treatments against salmon lice in marine salmonid farming, Aquaculture, Volume 575, 2023, 39749, ISSN 0044-8486, https://doi.org/10.1016/j.aquaculture.2023.739749.
Barrett, L.T., Oppedal, F., Robinson, N. and Dempster, T. (2020), Prevention not cure: a review of methods to avoid sea lice infestations in salmon aquaculture. Rev. Aquacult., 12: 2527-2543. https://doi.org/10.1111/raq.12456
Barrett, L. T., Overton, K., Stien, L. H., Oppedal, F., & Dempster, T. (2020b). Effect of cleaner fish on sea lice in Norwegian salmon aquaculture: a national scale data analysis. International journal for parasitology, 50(10-11), 787-796.
Brooker, A.J., Papadopoulou, A., Gutierrez, C., Rey, S., Davie, A. and Migaud, H. (2018), Sustainable production and use of cleaner fish for the biological control of sea lice: recent advances and current challenges. Veterinary Record, 183: 383-383. https://doi.org/10.1136/vr.104966
Cáceres, P., Barría, A., Christensen, K.A. et al. Genome-scale comparative analysis for host resistance against sea lice between Atlantic salmon and rainbow trout. Sci Rep 11, 13231 (2021). https://doi.org/10.1038/s41598-021-92425-3
Casuso, A.; Valenzuela-Muñoz, V.; Benavente, B.P.; Valenzuela-Miranda, D.; Gallardo-Escárate, C. Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar). Vaccines 2022, 10, 1063. https://doi.org/10.3390/vaccines10071063
Coates, A., Robinson, N. A., Dempster, T., Johnsen, I., & Phillips, B. L. (2023). Evolutionary predictions for a parasite metapopulation: Modelling salmon louse resistance to pest controls in aquaculture. Evolutionary Applications, 00, 1–17. https://doi.org/10.1111/eva.13618
Gallardo-Escárate, C., Arriagada, G., Carrera, C., Gonçalves, A.T., Nuñez-Acuña, G., Valenzuela-Miranda, D. and Valenzuela-Muñoz, V. (2019), The race between host and sea lice in the Chilean salmon farming: a genomic approach. Rev Aquacult, 11: 325-339. https://doi.org/10.1111/raq.12334
Gentry K, Bui S, Oppedal F, Dempster T (2020) Sea lice prevention strategies affect cleaner fish delousing efficacy in commercial Atlantic salmon sea cages. Aquacult Environ Interact 12:67-80. https://doi.org/10.3354/aei00348
Hannisdal, R., Nøstbakken, O. J., Hove, H., Madsen, L., Horsberg, T. E., & Lunestad, B. T. (2020). Anti-sea lice agents in Norwegian aquaculture; surveillance, treatment trends and possible implications for food safety. Aquaculture, 521, 735044.
Hemmingsen, W., MacKenzie, K., Sagerup, K., Remen, M., Bloch-Hansen, K., & Imsland, A. K. D. (2020). Caligus elongatus and other sea lice of the genus Caligus as parasites of farmed salmonids: a review. Aquaculture, 522, 735160.
Leclercq, E., Pontefract, N., Rawling, M., Valdenegro, V., Aasum, E., Andujar, L. V., … & Merrifield, D. (2020). Dietary supplementation with a specific mannan-rich yeast parietal fraction enhances the gut and skin mucosal barriers of Atlantic salmon (Salmo salar) and reduces its susceptibility to sea lice (Lepeophtheirus salmonis). Aquaculture, 529, 735701.
Nagasawa K. 2004. Sea Lice, Lepeophtheirus salmonis and Caligus orientalis (Copepoda: Caligidae), of Wild and Farmed Fish in Sea and Brackish Waters of Japan and Adjacent Regions: A Review . Zoological Studies 43(2): 173-178.
Nilsen, A., Nielsen, K. V., Biering, E., & Bergheim, A. (2017). Effective protection against sea lice during the production of Atlantic salmon in floating enclosures. Aquaculture, 466, 41-50.
O’Donohoe P., S. Kennedy, F. Kane, O. Naughton, D. Tierney, L. Copley and D. Jackson. 2004. National Survey of Sea lice (Lepeophtheirus salmonis Krøyer and Caligus elongatus Nordmann) on Fish Farms in Ireland – 2003 . Aquaculture and Catchment Management Services, Marine Institute. 34 p.
Oldham Tina. 2023. Salinity and temperature alter the efficacy of salmon louse prevention, Aquaculture, Volume 575, 2023, 739673, ISSN 0044-8486, https://doi.org/10.1016/j.aquaculture.2023.739673.
Overton K, Barrett LT, Oppedal F, Kristiansen TS, Dempster T (2020) Sea lice removal by cleaner fish in salmon aquaculture: a review of the evidence base. Aquacult Environ Interact 12:31-44. https://doi.org/10.3354/aei00345
Parsons, A. E., Escobar-Lux, R. H., Sævik, P. N., Samuelsen, O. B., & Agnalt, A. L. (2020). The impact of anti-sea lice pesticides, azamethiphos and deltamethrin, on European lobster (Homarus gammarus) larvae in the Norwegian marine environment. Environmental Pollution, 264, 114725.
Powell, A., Treasurer, J.W., Pooley, C.L., Keay, A.J., Lloyd, R., Imsland, A.K. and Garcia de Leaniz, C. (2018), Use of lumpfish for sea-lice control in salmon farming: challenges and opportunities. Rev Aquacult, 10: 683-702. https://doi.org/10.1111/raq.12194
Robaldo R., J. Pereira, L. Sampaio, V. Kütter & A. Bianchini. 2002. Ovoposição e desenvolvimento inicial de Caligus sp. (Copepoda: Caligidae) parasita de juvenis do linguado Paralichthys orbignyanus (Teleostei: Paralichthyidae) em cativeiro. Atlântica, Rio Grande, 24(2): 85-88.
Robledo, D., Gutiérrez, A. P., Barría, A., Lhorente, J. P., Houston, R. D., & Yáñez, J. M. (2019). Discovery and functional annotation of quantitative trait loci affecting resistance to sea lice in Atlantic salmon. Frontiers in genetics, 10, 56.
Solé, M.; Lenoir, M.; Fortuño, J.-M.; De Vreese, S.; van der Schaar, M.; André, M. Sea Lice Are Sensitive to Low Frequency Sounds. J. Mar. Sci. Eng. 2021, 9, 765. https://doi.org/10.3390/jmse9070765
Todd C. 2006. The copepod parasite (Lepeophtheirus salmonisCaligus elongatus Nordmann) interactions between wild and farmed Atlantic salmon (Salmo salar L.) and wild sea trout (Salmo trutta L.): a mini review (Krøyer), . Journal of Plankton Research 29(1): 61-71.
Treasurer J. 2002. A review of potential pathogens of sea lice and the application of cleaner fish in biological control . Pest Management Science 58 (6): 546-558.
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.







