The projected expansion of the global aquaculture industry faces a significant hurdle: disease outbreaks caused by bacterial pathogens. Within this landscape, infections caused by Flavobacterium genus pathogens are persistent, particularly in salmonid aquaculture.
Traditionally, treatment has been limited to the use of antimicrobials, a practice that entails serious risks: contamination of the aquatic environment and the promotion of Antimicrobial Resistance (AMR). Aquaculture has been labeled a “hot spot” for the evolution of antibiotic resistance genes.
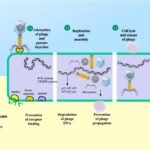
In light of this situation, research has shifted towards more sustainable and responsible alternatives. This is where bacteriophages (or phages) emerge—viruses that naturally attack and eliminate bacteria, offering a promising avenue for disease prevention and control.
This article, published by researchers from the University of Copenhagen, the University of Jyväskylä, the Technical University of Denmark, and the University of Southern Denmark, breaks down the potential of phage therapy in aquaculture as a tool to combat key Flavobacterium pathogens such as F. columnare and F. psychrophilum.
Key findings
- Phage resistance in Flavobacterium pathogens (F. columnare and F. psychrophilum) is frequently associated with mutations in the Type IX Secretion System (T9SS). This resistance leads to a loss or reduction of pathogen virulence, positioning phages as a promising route for pathogen control.
- Phages that infect Flavobacterium demonstrate persistence to environmental and storage conditions, remaining active for years at 4 oC in liquid formulations and retaining infectivity in dry feed stored at 5 oC for up to 8 months. This makes them potentially suitable for the development of commercial products.
- There is experimental evidence that phage therapy can effectively control the pathogen at the laboratory scale. The most efficient impact is achieved by administering phages to the water as a bath treatment when the first symptoms appear, leading to an efficient reduction in the virulent bacterial population.
- Despite the potential, significant challenges persist that limit the development of viable commercial products. These include the need for farm-scale testing, resolving regulatory complexity, optimizing industrial-scale production and delivery methods, and addressing the risk of adaptive resistance (such as CRISPR-Cas in F. columnare).
- Phage therapy offers an environmentally responsible approach that does not promote antimicrobial resistance, positioning it as an urgent and necessary alternative to antibiotic treatments, which contaminate the aquatic environment and promote resistance.
How phages work: A natural weapon
Phages have been investigated for their ability to prevent and control diseases and the formation of biofilms in aquaculture. Phages that infect Flavobacterium have already proven to be persistent to environmental and storage conditions, making them suitable for commercial product development.
The connection between phages, T9SS, and virulence loss
A crucial finding in the phage-host interaction is the relationship between phage resistance and the loss of pathogen virulence.
- Resistance Mechanism: Phages bind to specific receptors on the bacterial cell surface, such as outer membrane proteins or surface glycoconjugates. To evade infection, bacteria can mutate these receptors.
- The Role of T9SS: These mutations often affect genes associated with the Type IX Secretion System (T9SS). This complex system is vital for gliding motility and the secretion of enzymes and adhesins.
- The Trade-off: The T9SS also plays an essential role in the virulence of F. columnare and F. psychrophilum. When a bacterium mutates T9SS components to become phage-resistant, this alteration comes at a biological cost: the gliding machinery is disrupted, protease secretion is affected, and changes in biofilm formation occur.
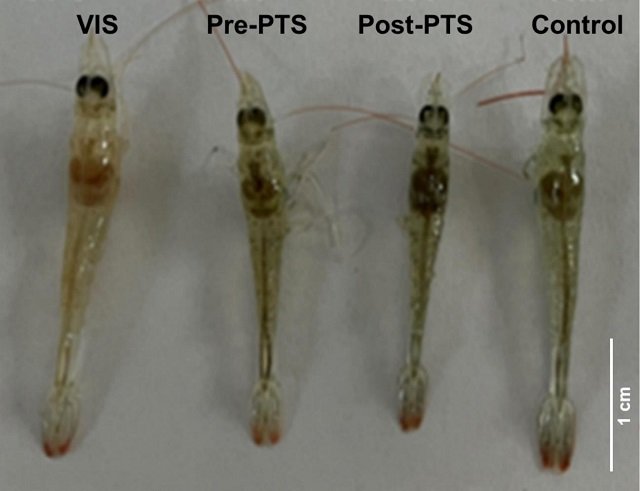
- Practical Implication: Phage-resistant isolates have shown a reduced or complete lack of ability to cause mortality in experimentally infected rainbow trout and zebrafish. This suggests that, even if the pathogen develops resistance, the disease control objective is achieved by transforming it into a harmless or attenuated strain.
Diversity and scope of Flavobacterium phages
The study reports that more than 280 phages that infect F. columnare and F. psychrophilum have been isolated and published. The host range is a key consideration for phage therapy, as phages must be able to infect the diversity of strains present on a farm.
- High Infection Success: Phage collections have successfully infected a high percentage of pathogen isolates: up to 98% of F. psychrophilum isolates globally and 94% of F. columnare isolates.
- Evolutionary Adaptation: Antagonistic coevolution between phages and bacteria is one of the fastest evolutionary processes. Aquaculture studies have shown that bacteria develop resistance to phages from past years but are less resistant to more recent phages. This underscores the need for constant isolation and the use of the “phage training” technique to expand their host range against resistant strains.
The challenge of CRISPR-Cas resistance
Phages are the natural countermeasure to bacteria, but bacteria also have their defenses. In addition to surface mutations, bacteria possess adaptive immunity systems like CRISPR-Cas.
- In F. columnare: This species has two active CRISPR loci (subtypes II-C and VI-B) that acquire “memory” (spacers) in response to phage exposure, confirming their active involvement in phage defense in natural populations.
- In F. psychrophilum: Although CRISPR systems have been identified, the stability of their spacer composition suggests it is not a dynamic and active defense mechanism to the same extent as surface mutations.
In this context, the biological control strategy appears more promising in F. psychrophilum, where resistance is usually exclusively due to surface modification (resulting in loss of virulence). In contrast, the ability of F. columnare to use adaptive CRISPR systems could make the long-term maintenance of a phage-based control strategy difficult without adjustments.
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
Delivery methods and stability: Getting phages to the fish
The success of phage therapy depends on an effective delivery method, which must be adapted to the pathogen’s infection route.
Proven methods:
- Bath Treatments: This is the most suitable strategy for pathogens that colonize external surfaces like gills (F. branchiophilum, F. columnare). Bath treatments have shown promising control of bacterial colonization and mortality.
- Oral Administration (Feed): For F. psychrophilum, which causes systemic infection, feed infused with phages has succeeded in delivering infective phages to the intestine and spleen. However, effectiveness is limited, possibly due to insufficient concentration in the feed or inactivation in the acidic stomach of the rainbow trout (average pH of 4.9 during feeding).
- Biofilters and Biofilms: F. columnare phages preferentially cluster in biofilms attached to biofilter media in Recirculating Aquaculture Systems (RAS), acting as reservoirs and maintaining effectiveness for at least 21 days. This suggests that the immobilization of phages on biomaterials (like biofilters) could be a viable prevention strategy to reduce the pathogen load in the system.
Stability:
- Phages that attack Flavobacterium are stable under neutral pH conditions (7.0-7.5) and have notable temperature and desiccation tolerance. Purified lysates can remain active for several years at 4 oC. Phages in feed have maintained between 10% and 60% infectivity after 8 months of storage at 5 oC and -80 oC, respectively.
Outstanding challenges: From research to market
Despite the potential, phage therapy in aquaculture still faces key challenges for commercialization:
- Regulation: In the US and the EU, phage products are regulated according to their intended use (disease treatment, food additive, biopesticide), creating a complex landscape. Products intended for decontamination or as feed additives have simpler regulatory requirements than veterinary medicines.
- Scaling production: Current protocols are limited to laboratory-scale production. Industrial production must comply with Good Manufacturing Practices (GMP) standards that have not yet been developed for Flavobacterium phages.
- Safety and purity: Production involves the use of pathogenic bacteria, which could pose a risk of horizontal transfer of virulence genes. Furthermore, bacterial lysis releases metabolites and endotoxins that can be harmful to fish (mortality has been observed in rainbow trout exposed to crude lysates). Industrial-scale purification protocols need to be adapted.
- Farm-condition testing: Validation must move from challenge models with specific strains to farm-scale testing that simulates natural outbreaks and addresses the natural diversity of pathogens.
Conclusion
Research on the phage-Flavobacterium interaction has revealed considerable potential for phage therapy. The resistance mechanism that results in the loss of virulence is a unique strength that suggests phage therapy can control the disease, regardless of bacterial resistance.
For this potential to translate into a commercially viable and sustainable solution, it is essential to address the challenges of regulation, large-scale production optimization, and the development of quality protocols. Phage therapy represents an essential tool for the resilience and responsible growth of aquaculture.
Contact
Mathias Middelboe
Marine Biology Section, Department of Biology, University of Copenhagen
Helsingør, Denmark
Department of Biology, University of Southern Denmark
Odense, Denmark
Email: mmiddelboe@bio.ku.dk
Reference (open access)
L. A. I. Landor, V. Ruffo, L.-R. Sundberg, L. Madsen, and M. Middelboe, “Phage-Host Interactions in Flavobacterium Pathogens and the Potential of Phage-Based Disease Control in Salmonid Aquaculture,” Reviews in Aquaculture 18, no. 1 (2026): e70115, https://doi.org/10.1111/raq.70115.
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.