Integrated Multi-Trophic Aquaculture (IMTA) emerges as a promising solution to aquaculture’s environmental challenges. By cultivating extractive species, such as kelp (Saccharina latissima), alongside fed species like Atlantic salmon (Salmo salar), this approach seeks to diversify production and reduce environmental impact through bioremediation.
However, scaling IMTA commercially is complex. The primary challenge is synchronization: nutrients released by the fish must be available precisely when the algae can efficiently absorb them. This alignment is affected by weather, currents, and production cycles.
Is it better to have more salmon? Or to seed the kelp at a different time? To answer this, a recent study by researchers from the University of Stirling’s Institute of Aquaculture and Bantry Marine Research Station Ltd. developed an integrated modeling approach to optimize seeding strategies at a commercial salmon and kelp farm in Bantry Bay, Ireland. The results challenge common assumptions and indicate a clear path toward optimization.
Key findings
- A new modeling study analyzed a commercial salmon and kelp IMTA system in Bantry Bay, Ireland.
- The limiting factor for kelp (Saccharina latissima) growth was not salmon-derived nitrogen, but light availability.
- Increasing salmon production (and its waste) did not significantly improve kelp growth or bioremediation.
- The key was advancing the kelp seeding (from winter to autumn) and extending its cultivation cycle (from 89 to 151 days), which quadrupled biomass and nitrogen removal.
- This optimized strategy would allow a 10% nitrogen bioremediation target to be met using only 36 cultivation lines, a feasible area within the farm’s current license.
The “Digital Lab” for simulating the farm
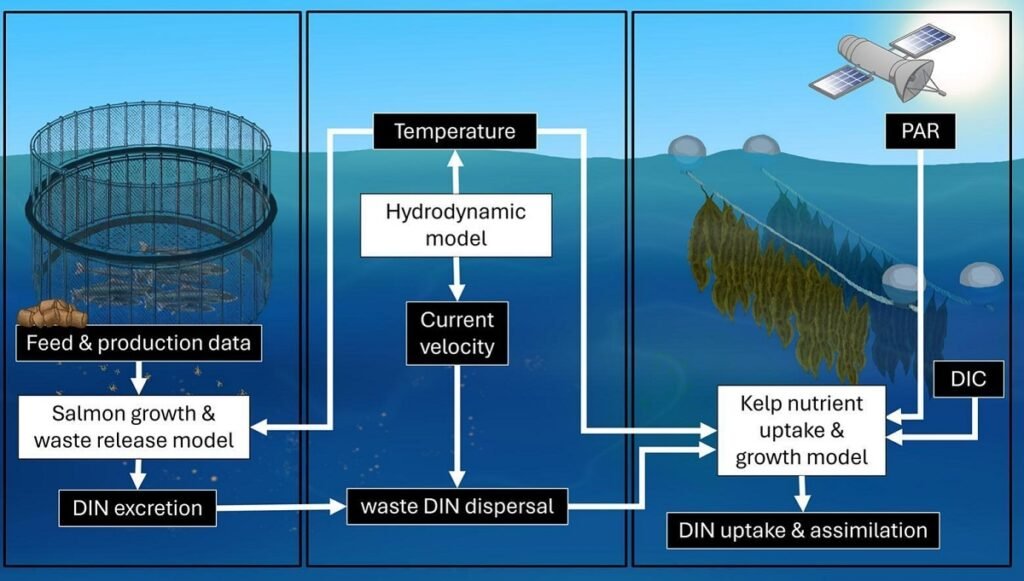
To understand the complex interaction between the fish, the environment, and the algae, the researchers combined three distinct models to create a comprehensive simulation of the farm.
The three-part model
The integrated approach functioned as follows:
- Salmon Model (FEEDNETICS): This model simulated salmon growth in the cages. Based on production, feeding, and temperature data, it calculated the daily amount of dissolved inorganic nitrogen (DIN) waste released by the fish into the environment.
- Hydrodynamic Model (ROMS): Once the nitrogen was released, this model simulated how marine currents and temperature in Bantry Bay transported these nutrients from the salmon cages to the kelp cultivation area, located approximately 200 meters away.
- Kelp Model (DEB): Utilizing a re-parameterized Dynamic Energy Budget (DEB) Model, this component simulated kelp growth. It calculated how the algae absorbed available nitrogen (from salmon and the background environment) and carbon (DIC), based on light (PAR) and temperature, to generate biomass.
This “digital lab” allowed researchers to virtually test different management strategies (scenarios) to determine which produced the most kelp and removed the most nitrogen.
Testing the scenarios: More salmon or more time?
The researchers applied their model to four distinct production scenarios to compare their effects:
- Scenario 1 (Baseline): The farm’s current practice. Salmon stocked in summer (June) and kelp seeded in winter (January), with an 89-day cultivation cycle for the kelp.
- Scenario 2 (More Salmon): Same seeding times, but increasing salmon production (nearly tripling metabolic nitrogen waste, from 15.35 t to 40.45 t).
- Scenario 3 (Advance Kelp): Same salmon, but seeding the kelp much earlier, in autumn (November), and extending its cultivation cycle to 151 days.
- Scenario 4 (Change Salmon): Same kelp (winter seeding), but stocking the salmon in autumn (October) to better align their peak waste production with kelp growth.
Nitrogen is not the limit; light is
The study’s findings were revealing, dismantling the idea that “more waste equals more kelp growth.”
Increasing salmon did not improve Kelp growth
The comparison of Scenarios 1, 2, and 4 showed insignificant differences in kelp yield. Final kelp biomass was nearly identical across all three scenarios (approx. 8.71 t).
Stay Always Informed
Join our communities to instantly receive the most important news, reports, and analysis from the aquaculture industry.
Surprisingly, tripling the available nitrogen (Scenario 2) did not result in greater kelp growth or higher nitrogen assimilation. The bioremediation percentage actually dropped drastically (from 0.71% to 0.27%), as there was far too much nitrogen for the same amount of kelp.
The researchers’ conclusion was clear: at this site, kelp growth is not limited by nitrogen availability. Even in the baseline scenario, nitrate levels often exceeded the kelp’s uptake saturation point. The true limiting factor, especially during winter months, was light availability (irradiance).
Kelp timing changes everything (Scenario 3)
The drastic change was observed in Scenario 3, where the kelp strategy was modified. By seeding the algae in November (autumn) and allowing a 151-day cultivation cycle, the results were radically different:
- Higher Biomass: Total farm kelp yield quadrupled, increasing from 8.71 t to 32.71 t.
- Improved Bioremediation: Net nitrogen assimilation also increased more than fourfold, reaching 471.44 kg. This represents 3.07% of the salmon’s metabolic waste (compared to just 0.71% in the baseline scenario).
Although initial kelp growth in November was slower (due to low winter light), this extra time was crucial. It allowed the algae to develop greater biomass and surface area, positioning them to enter their exponential growth phase when light conditions improved in March and April. The other scenarios harvested the kelp just before this growth “boom” could occur.
Practical implications: How much Kelp is needed for meaningful bioremediation?
The study went a step further, calculating the scale of kelp cultivation required to mitigate a modest target of 10% of the salmon’s metabolic nitrogen.
- In the Baseline Scenario (winter), 155 kelp lines (110 m each) would be required, occupying an area of nearly 35 hectares.
- If salmon were increased (Scenario 2), the demand would skyrocket to 401 lines (84 hectares).
- However, under Scenario 3 (autumn seeding), only 36 lines would be needed to achieve the same 10% bioremediation, occupying just 6.14 hectares.
This is the most relevant finding for the producer: the optimized strategy (36 lines) is not only more efficient but also fits within the farm’s current kelp license area (approx. 6 ha).
Despite this spatial feasibility, the authors note that scaling production (the 36 optimized lines would yield 107 t of kelp) would require substantial increases in national harvesting, processing, and drying capacity, which are currently a bottleneck.
Conclusion
This study demonstrates that in open-water salmon and kelp IMTA systems, such as in Bantry Bay, optimization fundamentally depends on the kelp strategy, not the salmon.
The limiting factor is not the nutrient (which is in surplus), but light. The simple act of advancing kelp seeding from winter to autumn and extending the cultivation cycle allows the algae to reach their exponential growth phase, quadrupling biomass and bioremediation efficiency.
This integrated modeling approach proves to be an invaluable management tool, enabling producers to test and optimize seeding strategies before in-water implementation, thereby saving time and enhancing IMTA’s sustainability and profitability.
Contact
Amalia Krupandan
Institute of Aquaculture, University of Stirling
Stirling, Scotland FK9 4LA, UK
Email: amalia.krupandan@stir.ac.uk
Reference (open access)
Krupandan, A., Falconer, L., Maguire, J., & Telfer, T. (2026). An integrated modelling approach for the stocking optimisation of a commercial-scale salmon-kelp IMTA system. Aquaculture, 613, 743299. https://doi.org/10.1016/j.aquaculture.2025.743299
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.