
The sea lamprey (Petromyzon marinus) is a fascinating yet troublesome species that has garnered attention due to its significant impact on aquatic ecosystems, particularly in the Great Lakes region. This primitive, jawless fish belongs to the Petromyzontidae family and is famous for its parasitic feeding habits, earning it a reputation as one of the most destructive invasive species.
Despite its poor reputation in North America, lampreys are considered a delicacy in some parts of Europe and are available seasonally in France, Spain, and Portugal. However, overfishing and river pollution are endangering sea lamprey populations, prompting efforts to recover these fish.
Embark on a captivating journey into the enigmatic world of the sea lamprey. Discover its fascinating habitat, explore its complex life cycle, uncover its unique diet, and the fascinating characteristics that distinguish it. Learn about its reproductive habits and understand the profound impact it has on the environment. Explore the various control methods employed to combat this formidable adversary and delve into the current challenges posed by this persistent parasite.
What is a Sea Lamprey?
The sea lamprey (Petromyzon marinus), a jawless fish with an elongated eel-like body, has gained notoriety for its parasitic lifestyle and the harmful impact it has inflicted on fish populations in the Great Lakes. This ancient creature, native to the Atlantic Ocean, has successfully invaded freshwater habitats, posing a significant threat to native fish species. Its parasitic nature, characterized by attaching to host fish and feeding on their blood, has caused substantial declines in fish populations, disrupting the delicate balance of the Great Lakes ecosystem.
Taxonomy of the Sea Lamprey
Domain: Eukaryota
Kingdom: Animalia
Phylum: Chordata
Subphylum: Agnatha
Class: Hyperoartia
Order: Petromyzontiformes
Family: Petromyzontidae
Genus: Petromyzon
Species: Petromyzon marinus Linnaeus, 1758
Common names: Sea lamprey, lake lamprey, vampire fish, lampern, suctorial, sucker
The Greek etymology of the scientific name refers to the behavior of adult lampreys attaching to rocks during nest building and mating, thus Petromyzon: petra, meaning “rock” or “stone”; and myzon, meaning “to suck.”
Characteristics
Several unique characteristics distinguish sea lampreys from other fish species. Their jawless mouth, circular suction disk, and parasitic feeding habits are among the most notable traits. Additionally, they possess a unique sensory system, including well-developed olfactory organs that aid in locating hosts.

Petromyzon marinus is the largest species of lamprey (Silva et al., 2019), typically measuring between 80 and 90 centimeters long, with weights of 2 to 3 kilograms. Their bodies are cylindrical and eel-like, with a dark gray or brown coloration. They have a circular, toothless mouth surrounded by a suction disk, which they use to attach to their hosts.
Vampire fish can live up to 11 years.
Distribution and Habitat: Revealing the aquatic realm of the sea lamprey
Sea lampreys are anadromous, jawless cartilaginous fish that are typically marine and ascend freshwater rivers to spawn; they can be found along the coasts of North America (Labrador and Gulf of Mexico) and the Atlantic coast of Europe and the Mediterranean Sea. However, Hume et al. (2021) highlight that due to climate change, the sea lamprey will likely be lost from the southern extent of its native distribution range but potentially expand northward as it tracks favorable habitats and hosts.
The vampire fish can be found at depths of up to 4000 meters and in temperature ranges of 1 to 20°C (34–68°F).
How do sea lampreys reproduce?
Upon reaching adulthood, sea lampreys migrate to spawn. During this spawning migration, they undergo significant physical changes, including the development of a large crest on their back and a transformation in their coloration. It is important to note that the sea lamprey does not exhibit sexual dimorphism until individuals return to the river to reproduce. According to Silva et al. (2019), the differences increase as sexual maturation progresses; thus, mature males are characterized by a marked dorsal midline thickening resembling a rope and by the projection of the urogenital papilla, while females develop a pseudo-fin between the anus and the caudal fin.
Female sea lampreys spawn in freshwater streams at a water temperature of at least 15°C, releasing a large number of eggs (from 150,000 to 300,000 eggs of <1 mm in diameter) into nests, which males then fertilize. Ammocoetes hatch after 7 to 14 days at temperatures between 15 and 25°C.

Production of sea lamprey larvae
Hume et al., (2024) experimented on the larval rearing of Petromyzon marinus, determining that:
- Water temperature: The highest average daily growth rates were recorded at 22 and 15°C (0.14 mm day−1) and the lowest at 8°C (0.06 mm day−1).
- Diet: Diets of yeast alone (0.19 and 0.21 g L−1) performed better than those comprising a mixture of yeast and other materials when fed 3 times a week (rice flour, wheat flour, fish flour; 0.19 and 0.32 g L−1).
- Stocking density: Culturing sea lamprey in captivity appears feasible at low density (31–32 g m−2 and 17–25 larvae m−2).
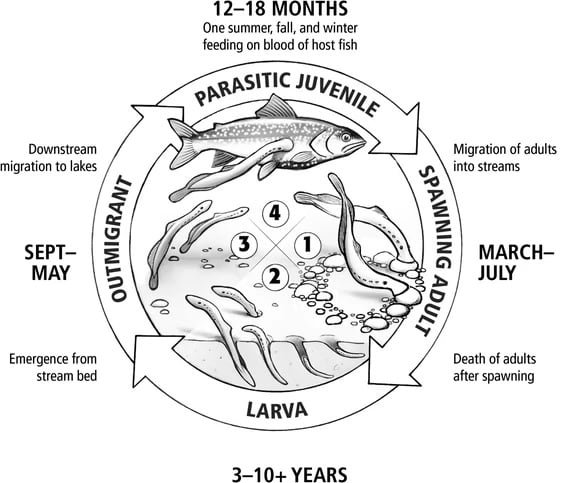
Life Cycle: A journey through the metamorphosis of the sea lamprey
The sea lamprey’s life cycle is a complex metamorphosis that passes through three distinct stages: ammocoete, transformed, and adult.
Ammocoete Stage: The early years of the sea lamprey’s life
The ammocoete stage is the larval phase of the vampire fish and lasts for several years. These filter feeders reside in stream beds, burrowing into sand or gravel. They are characterized by a slender, tadpole-like appearance, with gill slits on the sides of their bodies. During this phase, sea lamprey larvae primarily feed on microalgae of the genus Melosira and Navicula (Silva et al., 2019) and detritus.
Transformation Stage: Transitioning to adulthood
As ammocoetes mature, they undergo a transformation known as metamorphosis, transitioning to the transformation stage. This stage marks the development of adult characteristics, including the formation of eyes, nose, and fins. The transformation stage typically lasts for about one year.
Adult Stage: The parasitic phase of the sea lamprey
Upon reaching adulthood, sea lampreys emerge from their parasitic lifestyle and develop functional digestive and reproductive organs. They migrate to the Atlantic Ocean to spawn, a journey that can last several months. After spawning, adult sea lampreys die, completing their life cycle. Research findings by Silva et al. (2013), based on lampreys captured in Spain, suggest that at least a portion of the vampire fish population can reach adult size within 1 year of hematophagous feeding, indicating a period between completion of metamorphosis and reproduction of 1.5 years (18 to 20 months).
How does the sea lamprey feed?
Adult sea lampreys feed on the blood of fish (hematophagous feeding) and attach to their hosts using their suction disk. They then secrete an enzyme that dissolves the host’s skin, allowing them to feed on its blood and bodily fluids. This method of parasitic feeding has significantly contributed to the decline of fish populations in the Great Lakes.
During their larval stage, vampire fishes are filter feeders. Johnson et al. (2021a), using DNA metabarcoding of sea lamprey (Petromyzon marinus) fecal matter in the Laurentian Great Lakes, found them parasitizing fish such as lake trout (Salvelinus namaycush), lake whitefish (Coregonus clupeaformis), and longnose sucker (Catostomus catostomus).
Environmental Impact: The ecological footprint of the sea lamprey
The presence of sea lampreys in water bodies has serious economic implications, particularly for commercial and recreational fishing. Their parasitic nature not only reduces the abundance of native fish species but also affects their quality, making them less desirable for consumption.
The vampire fish Petromyzon marinus (Linnaeus) is a non-native invasive species in the Laurentian Great Lakes of North America and an endangered species in much of its native distribution area in North America and Europe (Hansen et al., 2016).
The introduction of sea lampreys into the Great Lakes has had a devastating impact on the region’s ecosystem. Their parasitic feeding habits have led to a significant decline in native fish populations, particularly lake trout, whitefish, and sturgeon. This ecological disruption has cascading effects throughout the food chain, affecting other aquatic species and the overall health of the ecosystem.
According to the Great Lakes Fishery Commission, vampire fish feed on most large fish species in the Great Lakes, including lake trout, brown trout, lake sturgeon, lake whitefish, cisco, burbot, yellow perch, catfish, and Pacific salmon, including chinook and coho salmon, and rainbow trout.
It is estimated that a sea lamprey can kill around 20 kilograms (40 pounds) of fish in a period of 12 to 18 months.
On the other hand, efforts to control sea lamprey populations incur significant costs for the agencies and government organizations involved in fisheries management.
Sea Lamprey Control Methods
Due to the harmful impact of sea lampreys on the Great Lakes ecosystem, several control methods have been implemented to manage their populations. These methods include:
Lampricides
Chemical agents that specifically target sea lampreys without harming other fish species. In this regard, Hlina et al. (2021) recommend considering water temperature when planning and executing lampricide applications to mitigate temperature-induced increases in vampire fish tolerance to 3-trifluoromethyl-4-nitrophenol (TFM) lampricide that could undermine sea lamprey control efforts in the Great Lakes. Moreover, Borowiec et al. (2022) reported that niclosamide was between 40 and 60 times more potent than TFM for lamprey control.
Chemical signals and pheromones
Buchinger et al. (2015) highlight that vampire fish use chemical signals and pheromones to identify spawning habitats, coordinate spawning behaviors, and avoid risks; and that manipulating olfactory biology offers opportunities for population management in the Laurentian Great Lakes. Additionally, research findings by Imre et al. (2014) support ongoing research on alarm signals generated by natural damages and predator repellents for manipulating the behavior of P. marinus populations.
Barriers
Physical barriers, such as dams and electric barriers, prevent sea lampreys from migrating upstream to spawning areas. Hansen et al. (2016) reported that traps within fish ladders are beneficial for removing sea lampreys in Great Lakes streams. Moreover, Johnson et al. (2021b) demonstrated that a seasonally deployed electric barrier can reduce the abundance of sea lamprey larvae, but effects on the movements and survival of other fish species remain a concern.
Predator restocking
Introducing natural predators, such as lake trout and salmon, that feed on sea lampreys.
However, Lennox et al. (2020) concluded that Great Lakes sea lampreys may benefit from climate change with longer growing seasons, faster growth, and increased access to spawning habitat; while Hume et al. (2021) forecast a reduction in pesticide efficacy and demand the rapid development of “more ecological” and less costly alternatives, but ecologically risky.
Economic Importance of the Sea Lamprey
Braga et al. (2020) reported that sea lamprey (Petromyzon marinus) fishing in the Miño River (Portugal) is carried out at night, with an average catch of larger sea lamprey of 2.6 kg; they also indicate that the price of this fishing resource could vary between 10 and 40 euros.
Beyond its nutritional importance as food, Dissanayake et al. (2021) reported that consumption of sea lamprey provides health benefits due to in vitro anti-inflammatory and antioxidant activities of isolates from this fish; they also indicate that the total content of fatty acids and glycerides in sea lamprey is similar to that of anchovy and mackerel.
Conclusion: The challenge of the sea lamprey
In conclusion, the sea lamprey presents a complex challenge that requires a multifaceted management approach. While efforts are needed to control their populations to protect native fish species and maintain ecosystem balance, it is essential to consider broader implications for the economy and livelihoods dependent on fishing. By achieving a balance between conservation and economic concerns, we can work towards sustainable solutions to mitigate the impact of the sea lamprey on aquatic ecosystems.
References
Borowiec, B. G., Birceanu, O., Wilson, J. M., McDonald, A. E., & Wilkie, M. P. (2022). Niclosamide is a much more potent toxicant of mitochondrial respiration than TFM in the invasive sea lamprey (Petromyzon marinus). Environmental Science & Technology, 56(8), 4970-4979.
Buchinger, T.J., Siefkes, M.J., Zielinski, B.S. et al. Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front Zool 12, 32 (2015). https://doi.org/10.1186/s12983-015-0126-9
Braga, H. O., Pereira, M. J., Musiello-Fernandes, J., Morgado, F., Soares, A. M., & Azeiteiro, U. M. (2020). The role of local ecological knowledge for the conservation and sustainable fisheries of the sea lamprey (Petromyzon marinus Linnaeus, 1758) in the Iberian Peninsula. Ocean & Coastal Management, 198, 105345. https://doi.org/10.1016/j.ocecoaman.2020.105345
Dissanayake, A. A., Wagner, C. M., & Nair, M. G. (2021). Evaluation of health benefits of sea lamprey (Petromyzon marinus) isolates using in vitro antiinflammatory and antioxidant assays. PLOS ONE, 16(11), e0259587. https://doi.org/10.1371/journal.pone.0259587
Hansen, M.J., Madenjian, C.P., Slade, J.W. et al. Population ecology of the sea lamprey (Petromyzon marinus) as an invasive species in the Laurentian Great Lakes and an imperiled species in Europe. Rev Fish Biol Fisheries 26, 509–535 (2016). https://doi.org/10.1007/s11160-016-9440-3
Hlina, B. L., Birceanu, O., Robinson, C. S., Dhiyebi, H., & Wilkie, M. P. (2021). The relationship between thermal physiology and lampricide sensitivity in larval sea lamprey (Petromyzon marinus). Journal of Great Lakes Research, 47, S272-S284. https://doi.org/10.1016/j.jglr.2021.10.002
Hume, J. B., Almeida, P. R., Buckley, C. M., Criger, L. A., Madenjian, C. P., Robinson, K. F., … & Muir, A. M. (2021). Managing native and non-native sea lamprey (Petromyzon marinus) through anthropogenic change: A prospective assessment of key threats and uncertainties. Journal of Great Lakes Research, 47, S704-S722.
Hume John B., Skyler Bennis, Tyler Bruning, Margaret F. Docker, Sara Good, Ralph Lampman, Jacques Rinchard, Trisha Searcy, Michael P. Wilkie, Nicholas S. Johnson, “Evaluation of Larval Sea Lamprey Petromyzon marinus Growth in the Laboratory: Influence of Temperature and Diet“, Aquaculture Research, vol. 2024, Article ID 5547340, 11 pages, 2024. https://doi.org/10.1155/2024/5547340
Imre, I., Di Rocco, R. T., Belanger, C. F., Brown, G. E., & Johnson, N. S. (2014). The behavioural response of adult Petromyzon marinus to damage-released alarm and predator cues. Journal of Fish Biology, 84(5), 1490-1502. https://doi.org/10.1111/jfb.12374
Johnson, N. S., Lewandoski, S. A., & Merkes, C. (2021a). Assessment of sea lamprey (Petromyzon marinus) diet using DNA metabarcoding of feces. Ecological Indicators, 125, 107605. https://doi.org/10.1016/j.ecolind.2021.107605
Johnson, N. S., Snow, B., Bruning, T., & Jubar, A. (2021b). A seasonal electric barrier blocks invasive adult sea lamprey (Petromyzon marinus) and reduces production of larvae. Journal of Great Lakes Research, 47, S310-S319. https://doi.org/10.1016/j.jglr.2021.09.008
Lennox, R. J., Bravener, G. A., Lin, Y., Madenjian, C. P., Muir, A. M., Remucal, C. K., Robinson, K. F., Rous, A. M., Siefkes, M. J., Wilkie, M. P., Zielinski, D. P., & Cooke, S. J. (2020). Potential changes to the biology and challenges to the management of invasive sea lamprey Petromyzon marinus in the Laurentian Great Lakes due to climate change. Global Change Biology, 26(3), 1118-1137. https://doi.org/10.1111/gcb.14957 https://onlinelibrary.wiley.com/doi/full/10.1111/gcb.14957
Silva S, Servia MJ, Vieira-Lanero R, Barca S, Cobo F (2013) Life cycle of the sea lamprey Petromyzon marinus: duration of and growth in the marine life stage. Aquat Biol 18:59-62. https://doi.org/10.3354/ab00488
Silva, S., Barca, S., Vieira-Lanero, R., Cobo, F. (2019). Lamprea marina – Petromyzon marinus. En: Enciclopedia Virtual de los Vertebrados Españoles. López, P., Martín, J., Cobo, F. (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/

