Food adulteration involves adding illegal or harmful substances to food to make them more appealing or extend their shelf life, a practice that has spread across all food-producing industries.
Formaldehyde, a colorless, pungent gas, is used in various industrial processes, including as a preservative in some foods, particularly in fish in developing countries. However, using formaldehyde to prolong the shelf life of food is illegal in many countries due to its known carcinogenic properties.
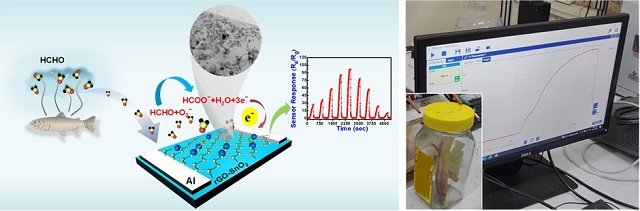
In this context, scientists from Gauhati University, the Indian Institute of Technology, and the Institute of Nano Science and Technology have developed a high-tech “sensor” capable of non-invasively detecting formalin adulteration in fish at room temperature. The sensor demonstrates long-term stability with a low detection limit.
Formaldehyde and Fish Adulteration
Due to the high demand for fish and seafood, the adulteration of these foods has become an unfair practice among fish traders to gain higher economic benefits.
The use of formaldehyde, known as “formalin,” in trade to extend the shelf life of fish and other foods is a problem in many countries. This compound is considered a carcinogen by the International Agency for Research on Cancer (IARC). Several studies suggest that adding formaldehyde to food is dangerous as it accumulates in the body.
Traders use formalin to extend the shelf life of fresh or frozen fish, artificially enhancing sensory attributes. The treatment of fish with formaldehyde reduces bacterial load and improves the textural properties of fish muscles, creating the impression that the fish is “fresh.” However, it’s important to note that formaldehyde can also be naturally produced in fish muscle, depending on various factors.
Ultrasensitive Formaldehyde Gas Sensor
Commercial formalin sensors for fish are mainly based on electrochemical or colorimetric methods. While electrochemical sensors are widely used, they are expensive and invasive. On the other hand, colorimetric sensors are less expensive but also invasive. Both methods face challenges related to low detection levels and selectivity.
The development of 2D gas sensors based on materials has created an effective way to detect toxic vapors at room temperature. These sensors have the potential to detect evaporated formaldehyde from adulterated food products.
Scientists have developed an ultrasensitive formaldehyde gas sensor made from a combination of tin oxide (SnO2) nanoparticles and reduced graphene oxide (rGO). This powerful duo, synthesized through a cost-effective wet chemical method, possesses exceptional capabilities:
- High Sensitivity: Detects formaldehyde at incredibly low levels, only 33 parts per billion (ppb), equivalent to a single drop in an Olympic-sized pool!
- Specificity: This sensor doesn’t get “fooled” by other odorous molecules. It specifically targets formaldehyde, ignoring unwanted distractions and ensuring accurate detection.
- Rapid Response: Recognizes formaldehyde instantly, with an ultrafast response time of 35 seconds even for minimal amounts.

An Ingenious Solution
Scientists have developed a cost-effective formalin sensor using a reduced graphene oxide-tin oxide compound that can effectively detect the presence of formalin in adulterated fish.
While graphene oxide (GO), the oxidized form of graphene, exhibits high processability in solution and ease of chemical modification with other materials, its low electrical conductivity poses a challenge. Scientists overcame this by developing the reduced graphene oxide-tin oxide (rGO-SnO2) compound.
While reduced graphene oxide (rGO) has been used to detect various toxic gases and VOCs, tin oxide (SnO2) has been extensively researched for formaldehyde detection either in pristine form or incorporated with various compounds, including graphene, due to its high stability and sensitivity to low concentrations of formaldehyde.
Impact on the Fish Trade
But what’s the real-world impact? This sensor isn’t just a laboratory boast. It has the potential to revolutionize food safety, particularly in the fight against fish contaminated with formaldehyde. Imagine non-invasive tests ensuring healthy seafood on our plates.
The sensor has been tested for detecting adulterated fish on a laboratory scale and in fish available in the fish markets of the Guwahati region. Advanced computer simulations confirmed that the presence of formaldehyde significantly alters the electronic charge distribution around the sensor, generating a clear and distinct signal. This understanding of the detection mechanism paves the way for further optimization and refinement of the sensor.
Conclusion
With this innovative technology, a future free from fraudulent practices and hidden formaldehyde contamination in fish, and food in general, is within our reach.
Scientists have designed a prototype that can be considered a breakthrough in the field of food adulteration. The prototype of this sensor will open new avenues for the development of affordable formalin sensor devices. Additionally, Mahata et al. (2024) report the use of the methodology and its optimization through machine learning.
This work paves the way for safer food, healthier consumers, and peace of mind with every meal.
Main Reference
Kashyap, A., Chakraborty, B., Siddiqui, M. S., Tyagi, H., & Kalita, H. (2023). Selective and Sensitive Detection of Formaldehyde at Room Temperature by Tin Oxide Nanoparticles/Reduced Graphene Oxide Composite. ACS Applied Nano Materials, 6(9), 7948-7959.
Other References
Mahata, B., Dixit, K., Acharyya, S., Banerji, P., & Guha, P. K. (2023). Adulterated fish recognition employing SnO 2 nanostructure-based chemiresistive sensor. IEEE Sensors Letters.
Mahata, B., Acharyya, S., Banerji, P., & Guha, P. K. (2024). Assessment of fish adulteration using SnO2 nanopetal-based gas sensor and machine learning. Food Chemistry, 438, 138039.
Mehta, N. K., Pal, D., Majumdar, R. K., Priyadarshini, M. B., Das, R., Debbarma, G., & Acharya, P. C. (2023). Effect of Artificial Formaldehyde Treatment on Textural Quality of Fish Muscles and Methods employed for Formaldehyde Reduction from Fish Muscles. Food Chemistry Advances, 100328.
Rahman, M. B., Hussain, M., Kabiraz, M. P., Nordin, N., Siddiqui, S. A., Bhowmik, S., & Begum, M. (2023). An update on formaldehyde adulteration in food: sources, detection, mechanisms, and risk assessment. Food chemistry, 136761.
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.
