The shrimp industry faces challenges due to various pathogens, including microsporidia such as Enterocytozoon hepatopenaei (EHP). This parasite infects the hepatopancreas (HP) and intestine of commercially important shrimp species like Penaeus vannamei and P. monodon, leading to growth retardation and reduced productivity.
Scientists from Mahidol University, the National Science and Technology Development Agency (NSTDA), and the University of Technology Tawan-ok conducted research confirming the pivotal role of EhPTP2 in the life cycle of EHP and demonstrated a successful method to disrupt it using RNA interference (RNAi) technology.
Enterocytozoon hepatopenaei (EHP)
Enterocytozoon hepatopenaei (EHP) infection does not directly cause high mortality but does result in reduced growth and increased feed consumption, ultimately affecting the profitability of shrimp farms. Studies have reported that EHP affects the microbiome of shrimp hepatopancreas.
EHP, like other microsporidia, possesses a resistant chitin wall, enabling them to survive in hostile environments for extended periods. Additionally, EHP has unique spores equipped with an invasion organelle called the polar tube. This microscopic structure allows the spore to invade host cells and initiate infection.
Targeting PTP2: A Promising Approach
The research focuses on a crucial component of the polar tube: polar tube protein 2 (PTP2), specifically the EHP variant (EhPTP2).
Researchers have identified similarities between EhPTP2 (the EHP version of PTP2) and its homologs in other microsporidia. Furthermore, studies suggest that EhPTP2 is located in the polar tube of germinated EHP spores. This localization hints at its crucial role in the infection process.
RNA Interference: Silencing the Enemy
RNA interference (RNAi) is a powerful tool that can silence specific genes. This technique has shown promise in controlling shrimp viruses. Researchers believe it can be adapted to combat microsporidia infections by targeting PTP genes.
Thus, the study aimed to investigate the potential of silencing EhPTP2 as a strategy to control EHP infection. Researchers hypothesized that inhibiting EhPTP2 function affects:
- Impeding EHP replication
- Reducing the number of EHP copies
- Limiting EHP transmission
Using a proteomic approach, the study aimed to identify proteins involved in spore germination. Additionally, researchers investigated spore extrusion sites along the shrimp digestive tract and the efficacy of specific EhPTP2-targeting RNAi to suppress EHP infection.
EhPTP2: The Key to Spore Extrusion?
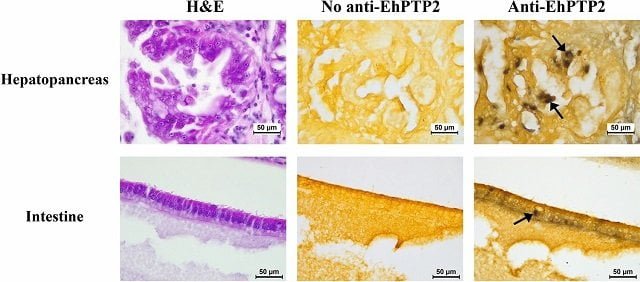
Based on this knowledge, scientists investigated proteins involved in extrusion. They discovered that EhPTP2 is abundant in extruded spores. Furthermore, using a special antibody that binds to EhPTP2, they confirmed the presence of extruded spores in the shrimp hepatopancreas (digestive gland) and intestine but not in the stomach, suggesting a specific target of the parasite.
Silencing EhPTP2 with RNAi
Researchers hypothesized that EhPTP2 might be essential for successful spore extrusion. To test this, they injected EHP-infected shrimp with dsRNA specifically targeting EhPTP2. This dsRNA activates RNAi, essentially silencing the EhPTP2 gene and preventing protein production.
The results were impressive. Shrimp injected with specific EhPTP2-targeting dsRNA showed a significant decrease in the number of EHP copies. This indicates that silencing EhPTP2 alters the parasite’s ability to amplify its numbers within the shrimp.
Transmission Blockade
The study didn’t stop there. Scientists also investigated the effect of specific EhPTP2-targeting dsRNA on EHP transmission. They injected infected shrimp with dsRNA before placing them with healthy, uninfected shrimp (cohabitation). The results were encouraging: the transmission rate of EHP to uninfected shrimp was significantly reduced.
Future Implications: A Brighter Future for the Shrimp Industry
The research reinforces the case for targeting EhPTP2 as a potential control method for EHP. It demonstrates that dsRNA can effectively reach and silence parasite genes within the shrimp host. This approach paves the way for further investigation into critical genes in the EHP life cycle, which could lead to the development of new preventive and therapeutic measures to safeguard shrimp health in aquaculture.
The study has been funded by Mahidol University, the National Science Research and Innovation Fund of Thailand.
Contact
Suparat Taengchaiyaphum
Aquatic Animal Health Research Team (AQHT), Integrative Aquaculture Biotechnology Research Group, National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency (NSTDA), Yothi Office, Rama VI Rd., Bangkok, 10400, Thailand
Email: suparat.tae@biotec.or.th
Chanadda Kasamechotchung
Department of Fisheries, Faculty of Agriculture and Natural Resources, Rajamangala University of Technology Tawan-ok, Chonburi, 20110, Thailand
Email: chanadda_ka@rmutto.ac.th
Reference (open access)
Yuanlae, S., Prasartset, T., Reamtong, O. et al. Shrimp injection with dsRNA targeting the microsporidian EHP polar tube protein reduces internal and external parasite amplification. Sci Rep 14, 4830 (2024). https://doi.org/10.1038/s41598-024-55400-2
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.
