The health of our fish farms is crucial, not only for our food security but also for the environment. Unfortunately, harmful bacteria such as Edwardsiella tarda and Vibrio harveyi can wreak havoc and cause diseases and even death among fish populations.
But there’s good news! A common disinfection method called chlorination proves effective in controlling these pathogens.
Recently, researchers from Seoul National University (Republic of Korea) delved into how chlorination affects specific bacteria in seawater environments. Their study explored how factors like pH levels, chlorine concentration, organic matter content, and water temperature influence the effectiveness of chlorination.
Two feared pathogens for fish farmers
Edwardsiella tarda is a Gram-negative bacterium affecting both freshwater and marine fish, causing a systemic disease called edwardsiellosis, characterized by symptoms of ascites, hernia, exophthalmia, and severe internal organ lesions (Xu & Zhang, 2014). Research has shown that phytobiotics can be employed to combat these bacteria.
Meanwhile, Vibrio harveyi is a pathogen affecting both fish and invertebrates. According to Zhang et al. (2020), sick fish may exhibit a variety of lesions, including ocular lesions/blindness, gastroenteritis, muscle necrosis, skin ulcers, and tail rot disease; while in shrimp, V. harveyi is considered the etiological agent of luminous vibriosis where affected animals glow in the dark.
Science in action: Unveiling the power of chlorination
The study investigated how different physical or chemical conditions of water affect chlorination’s ability to inactivate E. tarda and V. harveyi in seawater. They examined the impact of:
- Chlorine dosage: Higher chlorine concentration led to better inactivation.
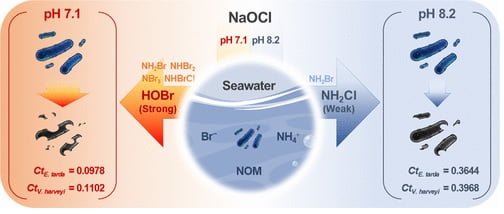
- Water pH: Lower pH (around 7.1) resulted in stronger disinfection compared to higher pH (around 8.2).
- Natural organic matter (NOM): The presence of NOM hindered chlorination effectiveness.
- Temperature: Warmer water facilitated quicker inactivation.
The power of chlorine-produced oxidants (CPO)
Chlorine alone doesn’t directly attack bacteria. Instead, it reacts with water to form CPO, the “true heroes” of this story. The type of CPO formed depends on pH and the presence of other elements like bromide and ammonium ions, commonly found in seawater.
The study found that at slightly acidic pH (7.1), a mix of potent CPO like HOBr (hypobromous acid) and NH2Cl (monochloramine) formed. HOBr is a superstar in killing bacteria, while NH2Cl is less effective. This explains why chlorination was more successful at this pH level.
Chlorination optimization for maximum impact
The study’s results revealed some fascinating insights:
- Chlorine power: Higher chlorine doses led to greater bacterial inactivation, making it a potent weapon.
- pH puzzle: Interestingly, a slightly acidic environment (pH 7.1) was more effective than a more alkaline one (pH 8.2). This is because the type of chlorine-produced oxidants (CPO) formed depends on pH. At pH 7.1, a mix of stronger bactericidal CPO dominated, while at pH 8.2, a weaker CPO took center stage.
- Organic obstacles: NOM, while natural, can hinder chlorination effectiveness. The study found that increasing NOM concentration reduced the bacteria destruction rate.
- Temperature matters: Warmer water temperatures improved chlorination efficiency, highlighting another environmental factor at play.
Ct: A crucial value for safeguarding fish
The study established crucial Ct values, a measure considering both chlorine concentration and exposure time. These Ct values indicate the minimum amount of chlorination needed to achieve a specific level of bacterial reduction (e.g., 99%).
For example, at pH 7.1, a Ct value of 0.0978 mg∙min/L was needed to reduce E. tarda by 99%. This value significantly increased to 0.3644 mg∙min/L when pH rose to 8.2, emphasizing the importance of maintaining a slightly acidic environment for optimal chlorination.
Conclusion: Guide to effective chlorination practices
“The Ct values determined in this study provide essential guidelines for safeguarding against microbial contaminants in seawater, thus proposing effective chlorination protocols in aquaculture,” conclude the researchers.
In this regard, this research provides valuable insights for the aquaculture industry. By understanding the factors influencing chlorination effectiveness and the importance of Ct values, fish farmers can implement more specific and efficient disinfection protocols. This not only safeguards fish health but also contributes to a more sustainable and productive aquaculture industry.
Reference
Cho, J., Kim, T., Cha, D., Lee, J. C., & Lee, C. (2024). Inactivation of Edwardsiella tarda and Vibrio harveyi by Chlorination in Seawater. ACS ES&T Engineering.
Others references
Xu, T., & Zhang, X. (2014). Edwardsiella tarda: An intriguing problem in aquaculture. Aquaculture, 431, 129-135. https://doi.org/10.1016/j.aquaculture.2013.12.001
Zhang, H., He, X., & Austin, B. (2020). Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Marine Life Science & Technology, 2(3), 231-245. https://doi.org/10.1007/s42995-020-00037-z
Editor at the digital magazine AquaHoy. He holds a degree in Aquaculture Biology from the National University of Santa (UNS) and a Master’s degree in Science and Innovation Management from the Polytechnic University of Valencia, with postgraduate diplomas in Business Innovation and Innovation Management. He possesses extensive experience in the aquaculture and fisheries sector, having led the Fisheries Innovation Unit of the National Program for Innovation in Fisheries and Aquaculture (PNIPA). He has served as a senior consultant in technology watch, an innovation project formulator and advisor, and a lecturer at UNS. He is a member of the Peruvian College of Biologists and was recognized by the World Aquaculture Society (WAS) in 2016 for his contribution to aquaculture.
